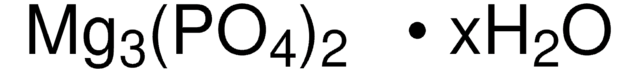677396
Magnesium aluminate, spinel
nanopowder, <50 nm particle size (BET)
Sinónimos:
Magnesium aluminate
About This Item
Productos recomendados
form
nanopowder
reaction suitability
reagent type: catalyst
core: magnesium
surface area
>30 m2/g
particle size
<50 nm (BET)
mp
2130 °C (lit.)
density
3.64 g/mL at 25 °C (lit.)
SMILES string
[Mg++].[O-][Al]=O.[O-][Al]=O
InChI
1S/2Al.Mg.4O/q;;+2;;;2*-1
InChI key
VPBIQXABTCDMAU-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
- A Review on Processing Polycrystalline Magnesium Aluminate Spinel (MgAl2O4): This review discusses various sintering techniques, material properties, and machinability of polycrystalline magnesium aluminate spinel, highlighting its optical transparency and mechanical properties (Z Shi et al., 2020).
- Effect of Structure and Composition of Non-Stoichiometry Magnesium Aluminate Spinel on Water Adsorption: This study examines how the structure and composition of magnesium aluminate spinel affect its water adsorption capabilities, focusing on the interaction with gases at different spinel compositions (Y Mordekovitz et al., 2020).
- Spectroscopic Study of Ordering in Non-Stoichiometric Magnesium Aluminate Spinel: The paper explores the ordering within non-stoichiometric magnesium aluminate spinel and its impact on material properties (V Erukhimovitch et al., 2015).
- Transparent Polycrystalline Magnesium Aluminate Spinel Fabricated by Spark Plasma Sintering: This research presents the fabrication of transparent polycrystalline magnesium aluminate spinel using spark plasma sintering, analyzing its mechanical and optical properties (M Sokol et al., 2018).
- A Comparative Study on the Effect of Different Additives on the Formation and Densification of Magnesium Aluminate Spinel: Investigates the effects of various additives on the formation and densification of magnesium aluminate spinel, providing insights into the synthesis and optimization of this ceramic material (SK Mohan et al., 2016).
Legal Information
signalword
Warning
hcodes
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
13 - Non Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico