529133
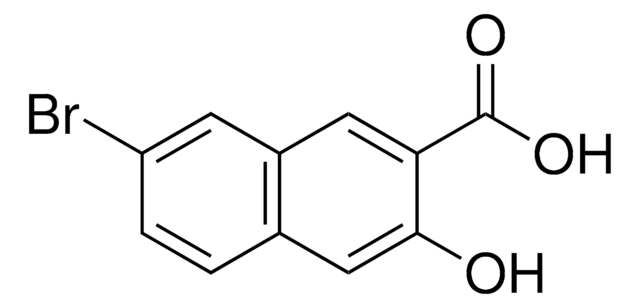
1-Methoxy-2-naphthoic acid
97%
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
CH3OC10H6CO2H
Número de CAS:
Peso molecular:
202.21
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
97%
mp
125-129 °C (lit.)
SMILES string
COc1c(ccc2ccccc12)C(O)=O
InChI
1S/C12H10O3/c1-15-11-9-5-3-2-4-8(9)6-7-10(11)12(13)14/h2-7H,1H3,(H,13,14)
InChI key
PMJACRPIWSINFF-UHFFFAOYSA-N
General description
1-Methoxy-2-naphthoic acid can be prepared from 1-methoxynaphthalene. 1-Methoxy-2-naphthoic acid can also be synthesized by reacting potassium tert-butoxide with 1-methoxynaphthalene and butyllithium in the presence of cyclohexane and tetrahydrofuran. It undergoes reduction in the presence of lithium to afford tetrahydronaphthoic acid.
Application
1-Methoxy-2-naphthoic acid may be used in the synthesis of 2-(1-methoxy-2-naphthyl)-4,4-dimethyl-2-oxazoline. It may also be used in the synthesis of the following compounds:
- 1-sec-butyl-2-naphthoic acid
- 1-tert-butyl-2-naphthoic acid
- 1-ethyl-2-naphthoic acid
- 1-vinyl-2-naphthoic acid
- 1-phenyl-2-naphthoic acid
- 1-(2,5-dimethylphenyl)-2-naphthoic acid
- 2′-methoxy-[1,1′-binaphthalene]-2-carboxylic acid
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Eva Castagnetti et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 8(4), 799-804 (2002-02-22)
Judged by its capacity to promote a hydrogen/metal permutation at an ortho position, the trifluoromethoxy group is superior to both the methoxy and trifluoromethyl groups. Moreover, like CF(3) and unlike OCH(3), OCF(3) exerts a long-range effect that still considerably lowers
Chemistry of aryloxazolines. Applications to the synthesis of lignan lactone derivatives.
Meyers AI and Avila WB.
The Journal of Organic Chemistry, 46(19), 3881-3886 (1981)
The metalation of 1-methoxynaphthalene with n-butyllithium.
Graybill BM and Shirley DA.
The Journal of Organic Chemistry, 31(4), 1221-1225 (1966)
Birch Reduction of 2-Naphthoic and of ortho-Methoxynaphthoic Acids.
Eliel EL and Hoover TE.
The Journal of Organic Chemistry, 24(7), 938-942 (1959)
Regadia Aissaoui et al.
The Journal of organic chemistry, 77(1), 718-724 (2011-11-24)
Substitution of an ortho-fluoro or methoxy group in 1- and 2-naphthoic acids furnishing substituted naphthoic acids occurs in good to excellent yields upon reaction with alkyl/vinyl/aryl organolithium and Grignard reagents, in the absence of a metal catalyst without the need
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico![Dichloro[(S,S)-ethylenebis(4,5,6,7-tetrahydro-1-indenyl)]zirconium(IV)](/deepweb/assets/sigmaaldrich/product/structures/124/554/8e617376-6d1e-4ab5-af24-cd94f4ba26d3/640/8e617376-6d1e-4ab5-af24-cd94f4ba26d3.png)



![Tris[N,N-bis(trimethylsilyl)amide]yttrium](/deepweb/assets/sigmaaldrich/product/structures/867/983/5b7cb7cd-8879-49e4-a9d7-29c52aaa82a0/640/5b7cb7cd-8879-49e4-a9d7-29c52aaa82a0.png)