527254
(Trimethylsilyl)diazomethane solution
2.0 M in diethyl ether
Sinónimos:
TMS-Diazomethane solution, (Diazomethyl)trimethylsilane
About This Item
Productos recomendados
concentración
2.0 M in diethyl ether
densidad
0.773 g/mL at 25 °C
temp. de almacenamiento
2-8°C
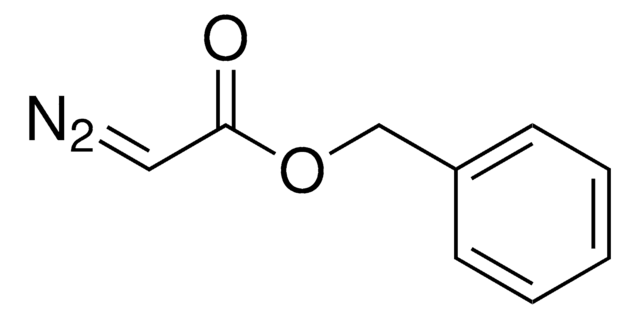
cadena SMILES
C[Si](C)(C)C=[N+]=[N-]
InChI
1S/C4H10N2Si/c1-7(2,3)4-6-5/h4H,1-3H3
Clave InChI
ONDSBJMLAHVLMI-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
Descripción general
Aplicación
- Macrolactam analogs of the natural product macrolide (-)-A26771B with improved metabolic stability and antibacterial activity
- Aigialomycin D analogues as protein kinase inhibitors for cancer treatment
- Unnatural α-amino acid derivatives containing gem-bisphosphonates via Michael addition reaction
- Capped 4-methylumbelliferyl hyaluronan disaccharides and tetrasaccharides as potential hyaluronidase substrates
- Stictamides A-C as matrix metallopeptidase 12 (MMP12) inhibitors with antitumor invasion activity
- Endothelin converting enzyme (ECE) Inhibitors WS 75624A and WS 75624B via a cross-metathesis approach
- Ent-kaurene derivatives as anti-inflammatory agents
- Desmosdumotin C analogs as potent antitumor agents acting via activation of spindle assembly checkpoint
- Imidazolo[2,1-b]benzothiazole derivatives as potential p53 inhibitors
Producto relacionado
Palabra de señalización
Danger
Frases de peligro
Consejos de prudencia
Clasificaciones de peligro
Acute Tox. 2 Inhalation - Acute Tox. 4 Oral - Carc. 1B - Flam. Liq. 2 - STOT SE 1 Inhalation - STOT SE 3
Órganos de actuación
Central nervous system, Lungs
Riesgos supl.
Código de clase de almacenamiento
3 - Flammable liquids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
-31.0 °F - closed cup
Punto de inflamabilidad (°C)
-35 °C - closed cup
Equipo de protección personal
Eyeshields, Faceshields, Gloves, type ABEK (EN14387) respirator filter
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico