479500
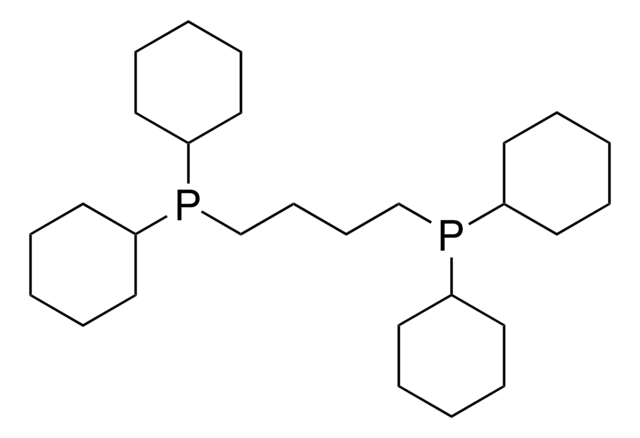
1,2-Bis(dicyclohexylphosphino)ethane
Sinónimos:
Ethylenebis(dicyclohexylphosphine), 1,2-Ethanediylbis[dicyclohexyl]phosphine
About This Item
Productos recomendados
form
solid
reaction suitability
reagent type: ligand
reaction type: Decarboxylations
reagent type: ligand
reaction type: Negishi Coupling
reagent type: ligand
reaction type: Suzuki-Miyaura Coupling
mp
92.5-96.5 °C (lit.)
functional group
phosphine
SMILES string
C1CCC(CC1)P(CCP(C2CCCCC2)C3CCCCC3)C4CCCCC4
InChI
1S/C26H48P2/c1-5-13-23(14-6-1)27(24-15-7-2-8-16-24)21-22-28(25-17-9-3-10-18-25)26-19-11-4-12-20-26/h23-26H,1-22H2
InChI key
BOUYBUIVMHNXQB-UHFFFAOYSA-N
Application
- Pd-catalyzed decarbonylative C-H coupling of azoles and aromatic esters.
- Ni-catalyzed cross-coupling reaction of aryl fluorides and primary amines.
- Investigations of the role of ligand-based steric effects during the polymerization
- Synthesis of molybdenum nitrosyl complexes for use as Imine hydrogenation catalysts
- Irreversible thermal linkage isomerization of switchable C-N-bound isomers
- Chelating for conversion of trans complexes to cis complexes
Precursor for Iridium trisboryl complexes and the substituent effect on borylation reactions
Ligand for palladium(II) complex catalyzed hydrogenation reactions
signalword
Danger
hcodes
Hazard Classifications
Aquatic Chronic 4 - Eye Dam. 1 - Skin Irrit. 2 - STOT SE 3
target_organs
Respiratory system
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
flash_point_f
>230.0 °F - closed cup
flash_point_c
> 110 °C - closed cup
ppe
Eyeshields, Gloves, type N95 (US)
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico








![[1,1′-Bis(di-cyclohexylphosphino)ferrocene]dichloropalladium(II) 98%](/deepweb/assets/sigmaaldrich/product/structures/136/854/a3142b2e-900c-47e5-8100-e48add9f4db6/640/a3142b2e-900c-47e5-8100-e48add9f4db6.png)