395307
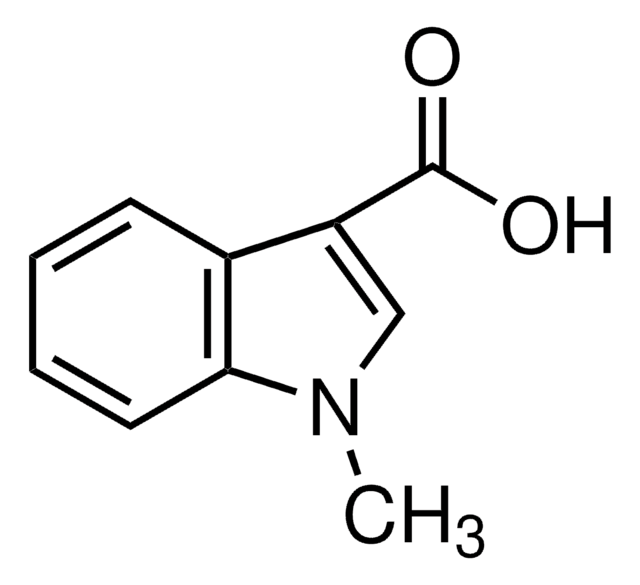
Methyl indole-3-carboxylate
99%
Sinónimos:
3-Carbomethoxyindole, 3-Methoxycarbonylindole, Methyl indolyl-3-carboxylate
About This Item
Productos recomendados
Ensayo
99%
Formulario
powder
mp
149-152 °C (lit.)
grupo funcional
ester
cadena SMILES
COC(=O)c1c[nH]c2ccccc12
InChI
1S/C10H9NO2/c1-13-10(12)8-6-11-9-5-3-2-4-7(8)9/h2-6,11H,1H3
Clave InChI
QXAUTQFAWKKNLM-UHFFFAOYSA-N
Descripción general
Aplicación
- Nitric oxide synthase (nNOS) inhibitorS
- Protein kinase c alpha (PKCα) inhibitors
- Inhibitors of the C-terminal domain of RNA polymerase II as antitumor agents
- Kinase insert domain receptor (KDR) inhibitors
- Organocatalysts for the anti-Mannich reaction
- Very late antigen-4 (VLA-4) antagonists
- Inhibitors of Human 5-Lipoxygenase
- Serotonin 5-HT4 receptor antagonists
- Hyaluronidase inhibitors
Reactant for:
- Enantioselective Meerwein-Eschenmoser Claisen rearrangement reactions†
Código de clase de almacenamiento
11 - Combustible Solids
Clase de riesgo para el agua (WGK)
WGK 3
Punto de inflamabilidad (°F)
Not applicable
Punto de inflamabilidad (°C)
Not applicable
Equipo de protección personal
dust mask type N95 (US), Eyeshields, Gloves
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Active Filters
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico