284491
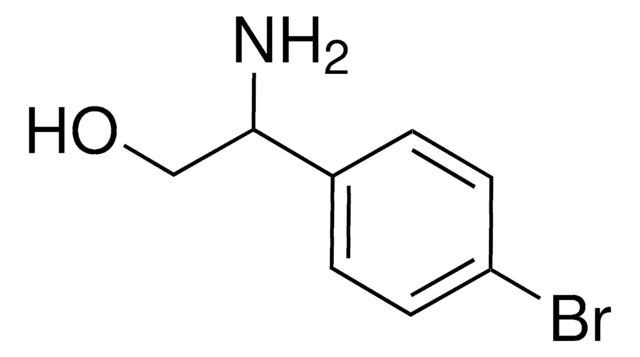
(R)-(+)-2-Amino-3-phenyl-1-propanol
98%
Sinónimos:
D-Phenylalaninol
Iniciar sesiónpara Ver la Fijación de precios por contrato y de la organización
About This Item
Fórmula lineal:
C6H5CH2CH(NH2)CH2OH
Número de CAS:
Peso molecular:
151.21
Beilstein/REAXYS Number:
2208239
EC Number:
MDL number:
UNSPSC Code:
12352116
PubChem Substance ID:
NACRES:
NA.22
Productos recomendados
assay
98%
form
solid
optical activity
[α]/D +22.8°, c = 1.2 in 1 M HCl
mp
93-95 °C (lit.)
functional group
amine
hydroxyl
phenyl
SMILES string
N[C@@H](CO)Cc1ccccc1
InChI
1S/C9H13NO/c10-9(7-11)6-8-4-2-1-3-5-8/h1-5,9,11H,6-7,10H2/t9-/m1/s1
InChI key
STVVMTBJNDTZBF-SECBINFHSA-N
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Elija entre una de las versiones más recientes:
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
M O Blondel et al.
Biochemical and biophysical research communications, 150(3), 979-986 (1988-02-15)
The illumination of Escherichia coli cells with UVA light, 320 nm less than or equal to lambda less than or equal to 380 nm, triggers a transient growth and division delay. The built-in 4-thiouridine chromophore which absorbs light at 340
W W Mak et al.
Canadian journal of biochemistry, 58(9), 737-744 (1980-09-01)
This study describes a rounding reaction induced in mammalian cells by the addition of phenylalaninol. In the Chinese hamster ovary tsH1 line the rounding occurred rapidly with a half time of 1 min at 25 mM phenylalaninol. After the removal
Violetta Constantinou-Kokotou et al.
Journal of peptide science : an official publication of the European Peptide Society, 11(7), 431-435 (2005-01-07)
2-Oxoamides based on long chain beta-amino acids were synthesized. 1-Benzyl substituted long chain amines, needed for such synthesis, were synthesized starting from Boc-phenylalaninol. The oxidative conversion of a phenyl group to a carboxyl group was used as the key transformation
B Weiss et al.
Research communications in chemical pathology and pharmacology, 62(1), 113-123 (1988-10-01)
An amino acid derivative, leucinethiol, was reported to be a strong inhibitor of aminopeptidase activity. In order to obtain selective inhibitors of various brain aminopeptidases, we tested the inhibition by amino acid analogs of brain aminopeptidase activity. In particular, we
H Hashizume et al.
Chemical & pharmaceutical bulletin, 40(11), 3113-3114 (1992-11-01)
The effects of phenylalaninol (D-isomer) on gastric acid secretion and gastric ulcer were studied in rats. The compound reduced the gastric acid secretion stimulated by intracisternal thyrotropin releasing hormone and intravenous 2-deoxy-D-glucose, but not that stimulated by subcutaneous carbachol or
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico
![Benzo[a]anthracene 99%](/deepweb/assets/sigmaaldrich/product/structures/351/486/b3ddf157-a732-4ef8-83f0-c70a53404cb2/640/b3ddf157-a732-4ef8-83f0-c70a53404cb2.png)


![Cyclopenta[d,e,f]phenanthrene 97%](/deepweb/assets/sigmaaldrich/product/structures/107/640/eed40ce0-e715-4438-9cb3-dc3dc13dcb9b/640/eed40ce0-e715-4438-9cb3-dc3dc13dcb9b.png)
![Benzo[b]fluoranthene 98%](/deepweb/assets/sigmaaldrich/product/structures/175/744/6fa5fca2-b6ec-47b6-ab7a-fe895843f226/640/6fa5fca2-b6ec-47b6-ab7a-fe895843f226.png)
![Benzo[a]pyrene ≥96% (HPLC)](/deepweb/assets/sigmaaldrich/product/structures/253/820/be96d879-1811-46c0-8f11-612019691c2d/640/be96d879-1811-46c0-8f11-612019691c2d.png)

![Benzo[ghi]perylene 98%](/deepweb/assets/sigmaaldrich/product/structures/154/740/c50ff1be-dfb4-4159-a98c-9cecf9206ad3/640/c50ff1be-dfb4-4159-a98c-9cecf9206ad3.png)