232211
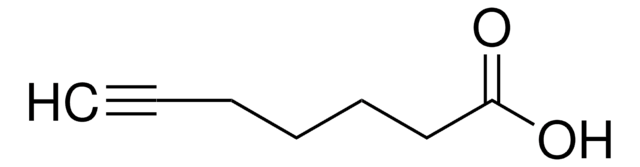
4-Pentynoic acid
95%
Sinónimos:
Propargylacetic acid
About This Item
Productos recomendados
assay
95%
form
solid
bp
110 °C/30 mmHg (lit.)
mp
54-57 °C (lit.)
storage temp.
2-8°C
SMILES string
OC(=O)CCC#C
InChI
1S/C5H6O2/c1-2-3-4-5(6)7/h1H,3-4H2,(H,6,7)
InChI key
MLBYLEUJXUBIJJ-UHFFFAOYSA-N
¿Está buscando productos similares? Visita Guía de comparación de productos
General description
Application
- as building block for the synthesis of library of eight sequence-defined model oligomers
- in one-pot synthesis of the complex polycyclic heterocycles benzo[4,5]imidazo[1,2-c]pyrrolo[1,2-a]quinazolinone derivatives
- in the synthesis of various allenenols lactones [5(E)-(2-allenylidene)-tetrahydro-2-furanones]
- in the synthesis of a cyctotoxic macrolide by ring-closing metathesis of a bis acetylene
signalword
Danger
hcodes
Hazard Classifications
Skin Corr. 1B
Storage Class
8A - Combustible corrosive hazardous materials
wgk_germany
WGK 3
flash_point_f
Not applicable
flash_point_c
Not applicable
ppe
Eyeshields, Faceshields, Gloves, type P3 (EN 143) respirator cartridges
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
Click chemistry, and the copper(I)-catalyzed azide-alkyne cycloaddition (CuAAC) in particular, is a powerful new synthetic tool in polymer chemistry and material science.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico