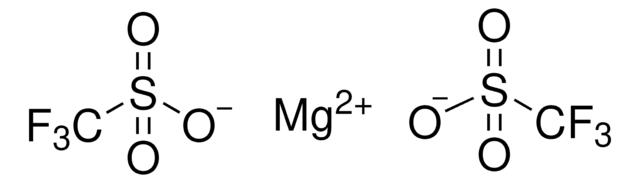176435
Silver trifluoromethanesulfonate
≥99%
Sinónimos:
Silver triflate, Trifluoromethanesulfonic acid silver salt
About This Item
Productos recomendados
Quality Level
assay
≥99%
form
powder
reaction suitability
core: silver
reagent type: catalyst
mp
286 °C (lit.)
SMILES string
[Ag+].[O-]S(=O)(=O)C(F)(F)F
InChI
1S/CHF3O3S.Ag/c2-1(3,4)8(5,6)7;/h(H,5,6,7);/q;+1/p-1
InChI key
QRUBYZBWAOOHSV-UHFFFAOYSA-M
¿Está buscando productos similares? Visita Guía de comparación de productos
Categorías relacionadas
Application
It can also be used:
- To obtain olefins from secondary phosphates and thiophosphates.
- As a reagent in the etherification of alcohols with primary alkyl halides under mild conditions.
- To generate cationic rhodium catalysts from chlororhodium complexes for the hydrophosphination of acetylenes.
- As a catalyst for the preparation of silyl ethers by hydrosilylation of aldehydes.
signalword
Danger
hcodes
Hazard Classifications
Eye Dam. 1 - Skin Irrit. 2
Storage Class
11 - Combustible Solids
wgk_germany
WGK 3
ppe
dust mask type N95 (US), Eyeshields, Gloves
Certificados de análisis (COA)
Busque Certificados de análisis (COA) introduciendo el número de lote del producto. Los números de lote se encuentran en la etiqueta del producto después de las palabras «Lot» o «Batch»
¿Ya tiene este producto?
Encuentre la documentación para los productos que ha comprado recientemente en la Biblioteca de documentos.
Los clientes también vieron
Artículos
We are proud to offer a treasure-trove of gold precatalysts and silver salts, as well as an extensive portfolio of unsaturated building blocks to accelerate your research success in this exciting field.
Nuestro equipo de científicos tiene experiencia en todas las áreas de investigación: Ciencias de la vida, Ciencia de los materiales, Síntesis química, Cromatografía, Analítica y muchas otras.
Póngase en contacto con el Servicio técnico