T7326
Thrombin from bovine plasma
≥60 NIH units/mg protein (biuret)
Synonym(s):
Factor IIa
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
CAS Number:
MDL number:
UNSPSC Code:
12352204
NACRES:
NA.54
Recommended Products
form
lyophilized powder
specific activity
≥60 NIH units/mg protein (biuret)
composition
Protein, ≥50%
storage temp.
−20°C
Looking for similar products? Visit Product Comparison Guide
General description
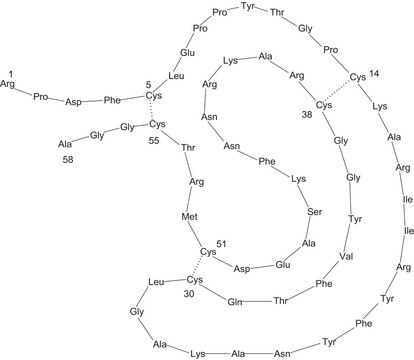
Thrombin is the final coagulation protease in regard to hemostasis, promoting both procoagulant and anticoagulant effects. Thrombin comprises of A-chain and B-chain with the catalytic pocket. The C-terminal regions has a fibrinogen recognition site (FRS) and a heparin binding site (HBS). The main function of thrombin is cleavage of fibrinogen to fibrin. It coordinates the coagulation cascade and coordinates with protease-activated receptors (PARs) to regulate physiological functions. High levels of thrombin causes neurotoxicity in dopaminergic neurons and contribute to the progression of parkinsons disease. Altered thrombin levels modulates coagulation pathway in multiple sclerosis. Patients with coronary artery disease (CAD) show elevated levels of thrombin.
Application
Thrombin from bovine plasma may be used:
as revitalization model to mimic blood fabrication in root canal
- for the generation of fibrin from fibrinogen embedded in microbubbles
- for the cleavage of hydrogenase maturation protein (HypF-N) from the nickel resin
as revitalization model to mimic blood fabrication in root canal
Thrombin is used for site specific cleavage of recombinant fusion proteins containing an accessible thrombin recognition site for removal of affinity tags. Thrombin has been used in a study to evaluate coagulation abnormalities in acute liver failure.
Biochem/physiol Actions
Serine protease that selectively cleaves Arg-Gly bonds in fibrinogen to form fibrin and fibrinopeptides A and B.
Unit Definition
Activity is expressed in NIH units obtained by direct comparison to a NIH thrombin reference standard.
Physical form
Lyophilized powder containing sodium chloride and Tris-HCl, pH 7.0
Preparation Note
Traditional thrombin products are activated with bovine brain thromboplastin, whereas T7326 is activated with bovine lung thromboplastin and does not contain any bovine brain products in its preparation.
Analysis Note
Activity is expressed in NIH units obtained by direct comparison to a NIH Thrombin Reference Standard, Lot K.
The NIH assay procedure uses 0.2 mL of diluted plasma (1:1 with saline) as a substrate and 0.1 mL of thrombin sample (stabilized in a 1% buffered albumin solution) based on a modification of the method of Biggs. Only clotting times in the range of 15-25 seconds are used for determining thrombin concentrations.
Other Notes
View more information on thrombin at www.sigma-aldrich.com/enzymeexplorer.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Fibrin-Targeted Polymerized Shell Microbubbles as Potential Theranostic Agents for Surgical Adhesions
Gormley CA, et al.
Langmuir (2019)
A training model for revitalization procedures
Widbiller M, et al.
International Endodontic Journal, 51, e301-e308 (2018)
Increased plasma thrombin potential is associated with stable coronary artery disease: An angiographically-controlled study
Tosi F, et al.
Thrombosis Research, 155, 16-22 (2017)
Banwari Agarwal et al.
Journal of hepatology, 57(4), 780-786 (2012-06-28)
In acute liver failure (ALF), prothrombin time (PT) and its derivative prothrombin time ratio (PTR) are elevated, and are considered predictors of increased bleeding risk. We aimed at determining whether increased PT/PTR reflects the haemostatic potential and bleeding risk in
Backbone NMR assignments of HypF-N under conditions generating toxic and non-toxic oligomers
Patel JR, et al.
Biomolecular NMR Assignments, 1-5 (2018)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service