10815
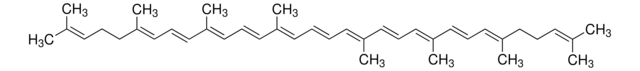
Ethyl β-apo-8′-carotenoate (trans)
≥80% (TLC)
Synonym(s):
Ethyl 8′-apo-β,ψ-caroten-8′-oate, all-trans-Carophyll yellow
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C32H44O2
CAS Number:
Molecular Weight:
460.69
Beilstein:
2021845
EC Number:
MDL number:
UNSPSC Code:
12352200
Recommended Products
Assay
≥80% (TLC)
impurities
≤0.001% heavy metals
ign. residue
≤0.1%
loss
≤0.1% loss on drying
mp
134-136 °C
solubility
chloroform: 10 mg/mL at 25 °C, clear, intense red-orange
storage temp.
−20°C
SMILES string
CCOC(=O)\C(C)=C\C=C\C(C)=C\C=C\C=C(C)\C=C\C=C(C)\C=C\C1=C(C)CCCC1(C)C
Other Notes
Internal standard in: Individual carotenoid determinations in human plasma by HPLC
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
C.C. Tangney
Journal of Liquid Chromatography, 7, 2611-2611 (1984)
A A Franke et al.
Journal of chromatography, 614(1), 43-57 (1993-04-21)
A method is described for the synthesis of new carotenoids by transesterification of ethyl-beta-apo-8'-carotenoate. The rate of transesterification was temperature-dependent with highest yields using primary alcohols. Reaction conditions were found avoiding Z isomerization. The structures of the new products were
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service