06054
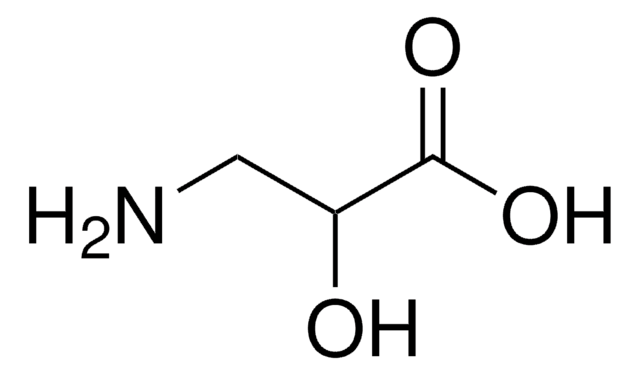
L-Isoserine
≥98.0% (TLC)
Synonym(s):
(S)-2-Hydroxy-β-alanine, (S)-3-Amino-2-hydroxypropionic acid
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C3H7NO3
CAS Number:
Molecular Weight:
105.09
MDL number:
UNSPSC Code:
12352209
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98.0% (TLC)
form
powder
reaction suitability
reaction type: solution phase peptide synthesis
color
white to off-white
mp
197-198 °C
application(s)
peptide synthesis
SMILES string
NC[C@H](O)C(O)=O
InChI
1S/C3H7NO3/c4-1-2(5)3(6)7/h2,5H,1,4H2,(H,6,7)/t2-/m0/s1
InChI key
BMYNFMYTOJXKLE-REOHCLBHSA-N
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yaqin Sun et al.
Frontiers in pharmacology, 12, 628209-628209 (2021-03-13)
Promoting the differentiation of oligodendrocyte precursor cells (OPCs) is important for fostering remyelination in multiple sclerosis. Catalpol has the potential to promote remyelination and exert neuroprotective effects, but its specific mechanism is still unclear. Recent studies have shown that the
B S Coller et al.
The Journal of biological chemistry, 268(28), 20741-20743 (1993-10-05)
Peptides containing sequences derived from the new NH2 terminus of the seven-transmembrane domain thrombin receptor after thrombin cleavage can activate platelets directly. We recently demonstrated that such peptides are readily cleaved and inactivated by plasma, serum, and endothelial cell-associated aminopeptidase
Thomas Rühl et al.
Chemical communications (Cambridge, England), (15)(15), 1630-1631 (2002-08-13)
The photolytic decomposition of trifunctional carbene generating photoaffinity probes in methanolic solution was studied, a cleavage reaction with butylamine in water, the conjugation with a ligand (moenomycin), and experiments that demonstrate that the fully armed probes interact with penicillin-binding protein
J Du et al.
Nucleosides & nucleotides, 17(1-3), 1-13 (1998-08-26)
Asymmetric synthesis of N-substituted oxazolidinyl nucleosides has been accomplished from L-isoserine, trans- and cis-Oxazolidine intermediates (4 and 5) were stereoselectively constructed from N-protected L-isoserine with a menthoxycarbonyl group by the condensation with benzoyloxy acetaldehyde dimethyl acetal in a ratio of
Nagarjuna Palyam et al.
The Journal of organic chemistry, 74(11), 4390-4392 (2009-05-02)
We report a new protocol for synthesis of L-1-deoxymannojirimycin, L-1-deoxyidonojirimycin, and the N-isopropyl derivative of the latter compound from the readily available precursors (S)-isoserinal hydrate and 2-tert-butyl-2-methyl-1,3-dioxan-5-one. The key steps include diastereoselective proline-catalyzed syn aldol transformation and a reductive amination/cyclization.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service