N1909
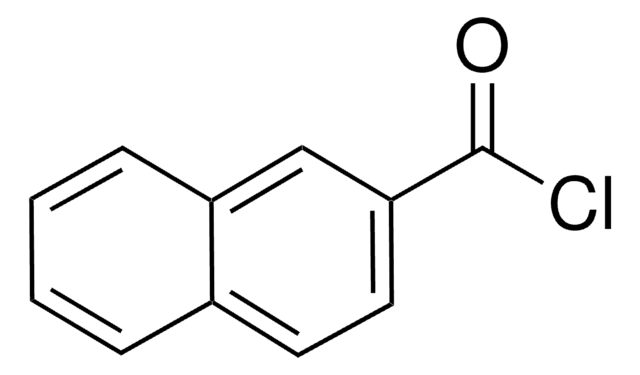
1-Naphthoic acid
96%
Synonym(s):
1-Naphthalenecarboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
C10H7CO2H
CAS Number:
Molecular Weight:
172.18
Beilstein:
1908896
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
96%
form
powder
bp
300 °C (lit.)
mp
157-160 °C (lit.)
SMILES string
OC(=O)c1cccc2ccccc12
InChI
1S/C11H8O2/c12-11(13)10-7-3-5-8-4-1-2-6-9(8)10/h1-7H,(H,12,13)
InChI key
LNETULKMXZVUST-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
1-Naphthoic acid can be used as a reactant to prepare:
- Perinaphthenones by dehydrative annulation with alkynes in the presence of rhodium catalyst.
- Isocoumarin derivatives by reacting with 2-butyne via aerobic oxidative cyclization using Rh catalyst.
- N-Methoxy-N-methyl-1-naphthalenecarboxamide (Weinreb amide) by reacting with N,O-dimethylhydroxylamine and phosphorus trichloride.
- 1,4-Dihydro-1-naphthalenecarboxylic acid by Birch reduction.
Other Notes
Remainder 2-naphthoic acid
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Qunfei Zhao et al.
Chemistry & biology, 15(7), 693-705 (2008-07-19)
Azinomycin B is a complex natural product containing densely assembled functionalities with potent antitumor activity. Cloning and sequence analysis of the azi gene cluster revealed an iterative type I polyketide synthase (PKS) gene, five nonribosomal peptide synthetases (NRPSs) genes and
S Chandra et al.
Spectrochimica acta. Part A, Molecular and biomolecular spectroscopy, 74(3), 704-713 (2009-09-02)
The Fourier transform infrared gas phase spectrum of Naphthoic acid (NA) was recorded in the region 4000-400 cm(-1). The Fourier transform Raman spectrum and Fourier transform IR spectra of NA were recorded in solid phase. Quantum chemical calculations of energies
Rajesh Sunasee et al.
The Journal of organic chemistry, 73(20), 8016-8020 (2008-09-26)
A method is described for converting tert-butyl benzoates or tert-butyl 1-naphthoates into derivatives having an alkyl or substituted alkyl group in a 1,4-relationship to an alkyl, aryl, alkenyl, or alkynyl group. Key steps in the sequence are (i) addition of
Mutual activation: Suzuki-Miyaura coupling through direct cleavage of the sp2 C-O bond of naphtholate.
Da-Gang Yu et al.
Angewandte Chemie (International ed. in English), 50(31), 7097-7100 (2011-06-29)
Hiromasa Uchiyama et al.
European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences, 43(1-2), 71-77 (2011-04-06)
Spray-dried particles (SDPs) with indomethacin (IND) and alpha-glycosyl transferase-treated stevia (Stevia-G) indicated extremely high dissolution rates and apparent solubility compared to particles of a ground mixture and a physical mixture of IND/Stevia-G. The apparent solubility of IND from SDPs was
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service