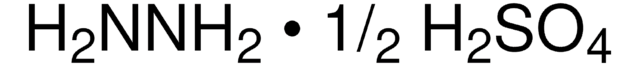901596
Hydrazine solution
1.0 M in ethanol
Synonym(s):
Hydrazine, Nitrogen hydride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2NH2
CAS Number:
Molecular Weight:
32.05
Beilstein:
878137
MDL number:
UNSPSC Code:
12352100
NACRES:
NA.22
Recommended Products
form
liquid
concentration
1.0 M in ethanol
refractive index
n/D 1.3666
density
0.7934 g/mL
functional group
hydrazine
SMILES string
NN
InChI
1S/H4N2/c1-2/h1-2H2
InChI key
OAKJQQAXSVQMHS-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Hydrazine promotes the cation exchange reaction for fast doping Cu ions into ZnSe nanocrystals.
Application
Valuable building block provided in ethanol for convenient handling.
Hydrazine solution may be employed for the following studies:
Hydrazine solution may be employed for the following studies:
- As reducing agent for the reduction of the first row (3d) transition metal complexes in aqueous solution.
- Preparation of nanoscale reduced metal oxides such as VO2(B), Cr2O3.
- As fuel for the direct hydrazine fuel cell (DHFC).
- As reducing agent for the synthesis of metallic monodisperse copper nanoparticles.
related product
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Aquatic Chronic 2 - Carc. 1B - Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - Skin Sens. 1
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
57.2 °F
Flash Point(C)
14 °C
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
M Vogel et al.
Fresenius' journal of analytical chemistry, 366(8), 781-791 (2001-03-03)
Hydrazine reagents are a well-known group of derivatizing agents for the determination of aldehydes and ketones in liquid and gaseous samples. Within this article, the most important hydrazine reagents are critically summarized, and their major applications in different fields, including
Nilay Hazari
Chemical Society reviews, 39(11), 4044-4056 (2010-06-24)
One of the most challenging problems in small molecule activation is the development of a homogeneous catalyst for converting dinitrogen into ammonia at ambient temperatures and atmospheric pressure. A catalytic cycle based on molybdenum that converts dinitrogen into ammonia has
D P Elder et al.
Journal of pharmaceutical and biomedical analysis, 54(5), 900-910 (2010-12-15)
This is the latest of a series of reviews focused on the analysis of genotoxic impurities. This review summarises the analytical approaches reported in the literature relating to hydrazine, hydrazines, hydrazides and hydrazones. It is intended to provide guidance for
Min Yuan et al.
Dalton transactions (Cambridge, England : 2003), (31)(31), 6078-6088 (2010-05-08)
A combination of unique solvent properties of hydrazine enables the direct dissolution of a range of metal chalcogenides at ambient temperature, rendering this an extraordinarily simple and soft synthetic approach to prepare new metal chalcogenide-based materials. The extended metal chalcogenide
Weidong Qu et al.
Biosensors & bioelectronics, 42, 430-433 (2012-12-19)
Graphene-nickel nanoparticle hybrid was prepared by the one-step far infrared-assisted reduction of graphene oxide and nickel (II) ions using hydrazine. It was loaded on the surface of a magnetic electrode for electrochemical sensing. The feasibility and performance of the novel
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service