556017
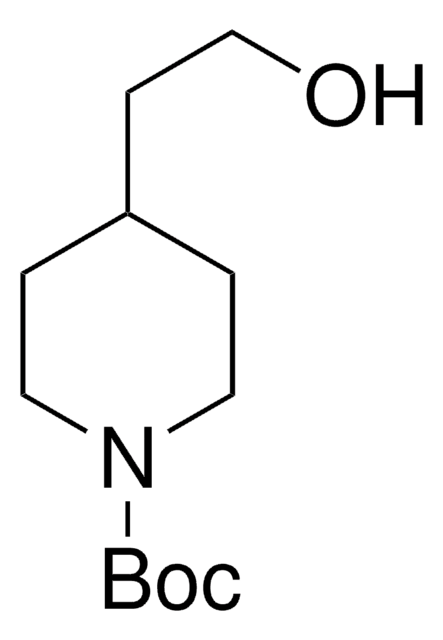
N-Boc-4-piperidinemethanol
97%
Synonym(s):
N-tert-Butyloxycarbonyl-4-piperidinemethanol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C11H21NO3
CAS Number:
Molecular Weight:
215.29
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
78-82 °C (lit.)
SMILES string
CC(C)(C)OC(=O)N1CCC(CO)CC1
InChI
1S/C11H21NO3/c1-11(2,3)15-10(14)12-6-4-9(8-13)5-7-12/h9,13H,4-8H2,1-3H3
InChI key
CTEDVGRUGMPBHE-UHFFFAOYSA-N
General description
N-Boc-4-piperidinemethanol contains a tert-butyloxycarbonyl (t-BOC)-protecting group. It can be synthesized from 4-piperidinemethanol via reaction with di-tert-butyldicarbonate.
Application
N-Boc-4-piperidinemethanol may be used to synthesize:
- methyl 5-methoxy-4-(1-methylpiperidin-4-ylmethoxy)-2-nitrobenzoate
- N-Boc-4-piperidinecarboxaldehyde
- [1-(tert-butoxycarbonyl)piperidin-4-yl]methyl methanesulfonate
Involved in the synthesis of bradycardic agents.
Storage Class Code
13 - Non Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and nanostructures of 5, 10, 15, 20-tetrakis (4-piperidyl) porphyrin.
JacobsenJL, et al.
Tetrahedron, 69(48), 10507-10515 (2013)
Fluorine-18 labeling of 6, 7-disubstituted anilinoquinazoline derivatives for positron emission tomography (PET) imaging of tyrosine kinase receptors: synthesis of 18F-Iressa and related molecular probes.
Seimbille Y, et al.
Journal of Labelled Compounds & Radiopharmaceuticals, 48(11), 829-843 (2005)
Hideki Kubota et al.
Bioorganic & medicinal chemistry letters, 14(12), 3049-3052 (2004-05-20)
A series of piperidinoalkanoyl-1,2,3,4-tetrahydroisoquinoline derivatives were synthesized, and their bradycardic activities were investigated in the isolated right atria of guinea pigs and in conscious rats. These efforts identified the achiral compound 2f, which exhibited potent and long-lasting bradycardic activity with
Daniele Zampieri et al.
European journal of medicinal chemistry, 44(1), 124-130 (2008-04-29)
We describe here the synthesis and the binding interaction with sigma(1) and sigma(2) receptors of a series of new benzo[d]oxazol-2(3H)-one derivatives variously substituted on the N-benzyl moiety. The results of binding studies confirm the notion that the benzoxazolone moiety confers
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service