All Photos(1)
About This Item
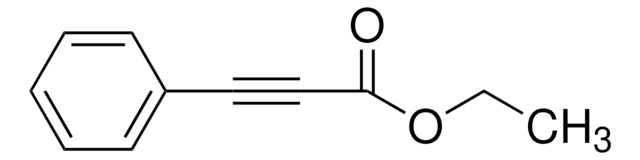
Linear Formula:
C6H5C≡CCO2CH3
CAS Number:
Molecular Weight:
160.17
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
refractive index
n20/D 1.5590 (lit.)
bp
109-112 °C/2 mmHg (lit.)
density
1.086 g/mL at 25 °C (lit.)
SMILES string
COC(=O)C#Cc1ccccc1
InChI
1S/C10H8O2/c1-12-10(11)8-7-9-5-3-2-4-6-9/h2-6H,1H3
InChI key
JFGWPXKGINUNDH-UHFFFAOYSA-N
Related Categories
General description
Organoiron carbonyl complexes are obtained by reacting methyl phenylpropiolate with Fe2(CO)9.
Application
Methyl phenylpropiolate may be used in the synthesis of:
- bicyclohexadienes
- cis-methyl cinnamate
- (E)-alkyl 3-(dialkoxyphosphoryl)-3-phenylacrylate derivatives
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation and Structures of Methyl Phenylpropiolate-Iron Carbonyl Complexes. A New Dicarbonyl-p-cyclopentadienyloxy-s-vinyliron Compound.
Dahl LF, et al.
Journal of the American Chemical Society, 88(3), 446-452 (1966)
P Andrew Evans et al.
Journal of the American Chemical Society, 127(36), 12466-12467 (2005-09-08)
Transition metal-catalyzed [m+n+o] carbocyclization reactions provide powerful methods for the construction of complex polycyclic systems that are generally not accessible through classical pericyclic reactions. We have developed the first regio- and enantioselective crossed intermolecular rhodium-catalyzed [2+2+2] carbocyclization of carbon- and
Steric effects on reactivity in silicon chemistry.
Cartledge FK.
Organometallics, 2(3), 425-430 (1983)
The catalyst-free addition of dialkyl phosphites on the triple bond of alkyl phenylpropiolates under microwave conditions.
Balint E, et al.
Current Catalysis, 4(1), 57-64 (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service