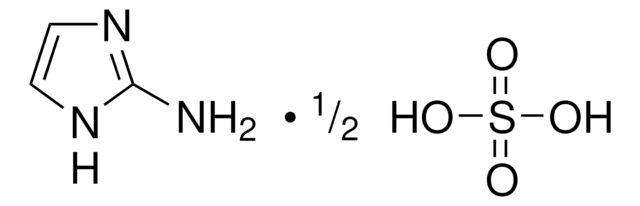All Photos(1)
About This Item
Linear Formula:
CF3OC6H4C(=NOH)NH2
CAS Number:
Molecular Weight:
220.15
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
113-115 °C (lit.)
SMILES string
N\C(=N/O)c1ccc(OC(F)(F)F)cc1
InChI
1S/C8H7F3N2O2/c9-8(10,11)15-6-3-1-5(2-4-6)7(12)13-14/h1-4,14H,(H2,12,13)
InChI key
COHKFOZYLCDVRK-UHFFFAOYSA-N
Related Categories
General description
4-(Trifluoromethoxy)benzamidoxime (4-TFMBAO) is a benzamidoxime (BAO) derivative containing amidoxime functional group. Its density and freezing point have been determined.
Application
4-(Trifluoromethoxy)benzamidoxime (4-TFMBAO) is suitable reactant in the fluorescence (FL) deriving reaction, one of the widely-used methodology specifically used to quantify uracil. It may be used as a reactant in the synthesis of oxadiazoles. It may also be used a fluorogenic agent in the quantification of orotic acid by spectrofluorometric method in human biological specimens.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Yaws CL.
The Yaws Handbook of Physical Properties for Hydrocarbons and Chemicals, 185-185 (2015)
Thomas E Barta et al.
Bioorganic & medicinal chemistry letters, 21(10), 2820-2822 (2011-04-22)
Seeking compounds preferentially potent and selective for MMP-13, we reported in the preceding Letter on a series of hydroxamic acids with a flexible benzamide tail groups.(1a) Here, we replace the amide moiety with non-hydrolyzable heterocycles in an effort to improve
Sensitive and Selective Determination of Orotic Acid in Biological Specimens Using a Novel Fluorogenic Reaction.
Yin S, et al.
Journal of Fluorescence, 25(4), 1005-1011 (2015)
Evan R Abt et al.
Cell chemical biology, 27(2), 197-205 (2019-11-18)
Biosynthesis of the pyrimidine nucleotide uridine monophosphate (UMP) is essential for cell proliferation and is achieved by the activity of convergent de novo and salvage metabolic pathways. Here we report the development and application of a cell-based metabolic modifier screening
Takayuki Shibata et al.
Analytica chimica acta, 674(2), 234-238 (2010-08-04)
Facile and specific methods to quantify a nucleobase in biological samples are of great importance for diagnosing disorders in nucleic acid metabolism. In the present study, a novel fluorogenic reaction specific for uracil has been developed. The reaction was carried
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service