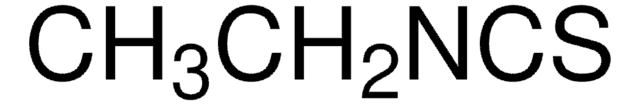All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H10N2O2S
CAS Number:
Molecular Weight:
186.23
Beilstein:
139617
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
mp
92-94 °C (lit.)
SMILES string
CCOC(=O)Cc1csc(N)n1
InChI
1S/C7H10N2O2S/c1-2-11-6(10)3-5-4-12-7(8)9-5/h4H,2-3H2,1H3,(H2,8,9)
InChI key
SHQNGLYXRFCPGZ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
Ethyl 2-aminothiazole-4-acetate is an organic ligand and possess strong coordination ability and display diverse coordination modes due to the presence of N, O coordination atoms.
Application
Ethyl 2-aminothiazole-4-acetate was used in the synthesis of:
- bis (2-aminothiazole-4-acetato)aquazinc(II)
- dichloridobis[ethyl 2-(2-amino-1, 3-thiazol-4-yl) acetate-κ2O,N3]cadmium
- novel heteroaryl-containing benzamide derivatives
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
365.0 °F - closed cup
Flash Point(C)
185 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Lai-Jun Zhang et al.
Acta crystallographica. Section E, Structure reports online, 68(Pt 6), m788-m789 (2012-06-22)
The asymmetric unit of the title compound, [CdCl(2)(C(7)H(10)N(2)O(2)S)(2)], contains two complex molecules with similar configurations. The Cd(II) atoms are each six-coordinated by two thiazole N and two carbonyl O atoms from the 2-(2-amino-1,3-thiazol-4-yl)acetate ligand, and by two Cl(-) anions in
Lai-Jun Zhang et al.
Acta crystallographica. Section E, Structure reports online, 65(Pt 12), m1517-m1517 (2009-01-01)
In the title compound, [Zn(C(5)H(5)N(2)O(2)S)(2)(H(2)O)], the central Zn atom (2 site symmetry) is five-coordinated by two N and three O atoms [Zn-N = 2.047 (3) Å, Zn-O = 2.099 (2) and 1.974 (4) Å] in a distorted square-pyramidal geometry. Besides one O atom from a
Kaapjoo Park et al.
Bioorganic & medicinal chemistry, 22(7), 2280-2293 (2014-03-05)
Novel heteroaryl-containing benzamide derivatives were synthesized and screened using an in vitro assay measuring increases in glucose uptake and glucokinase activity stimulated by 10mM glucose in rat hepatocytes. From a library of synthesized compounds, 3-(4-methanesulfonylphenoxy)-N-[1-(2-methoxy-ethoxymethyl)-1H-pyrazol-3-yl]-5-(3-methyl pyridin-2-yl)-benzamide (19e) was identified as
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service