All Photos(1)
About This Item
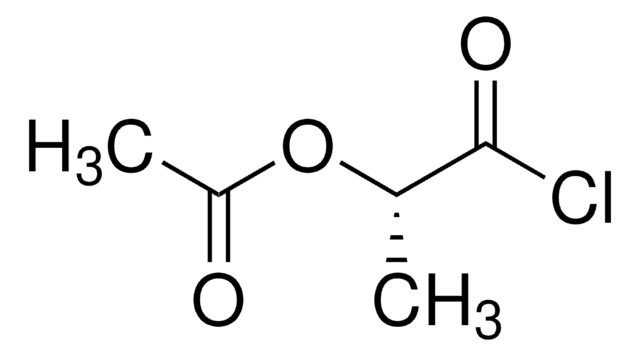
Linear Formula:
CH3COOCH(CH3)COOH
CAS Number:
Molecular Weight:
132.11
Beilstein:
1722938
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥97.0% (GC)
refractive index
n20/D 1.423
density
1.176 g/mL at 20 °C (lit.)
functional group
carboxylic acid
ester
SMILES string
CC(OC(C)=O)C(O)=O
InChI
1S/C5H8O4/c1-3(5(7)8)9-4(2)6/h3H,1-2H3,(H,7,8)
InChI key
WTLNOANVTIKPEE-UHFFFAOYSA-N
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Michael Vinogradov et al.
Analytical biochemistry, 342(1), 126-133 (2005-06-17)
Acetohydroxy acid synthase (AHAS) and related enzymes catalyze the production of chiral compounds [(S)-acetolactate, (S)-acetohydroxybutyrate, or (R)-phenylacetylcarbinol] from achiral substrates (pyruvate, 2-ketobutyrate, or benzaldehyde). The common methods for the determination of AHAS activity have shortcomings. The colorimetric method for detection
Ahmet Baykal et al.
Bioorganic chemistry, 34(6), 380-393 (2006-11-07)
In addition to the decarboxylation of 2-oxo acids, thiamin diphosphate (ThDP)-dependent decarboxylases/dehydrogenases can also carry out so-called carboligation reactions, where the central ThDP-bound enamine intermediate reacts with electrophilic substrates. For example, the enzyme yeast pyruvate decarboxylase (YPDC, from Saccharomyces cerevisiae)
N Goupil et al.
Applied and environmental microbiology, 62(7), 2636-2640 (1996-07-01)
Diacetyl is a by-product of pyruvate metabolism in Lactococcus lactis, where pyruvate is first converted to alpha-acetolactate, which is slowly decarboxylated to diacetyl in the presence of oxygen. L. lactis usually converts alpha-acetolactate to acetoin enzymatically, by alpha-acetolactate decarboxylase encoded
Gonzalo Jaña et al.
Proteins, 78(7), 1774-1788 (2010-03-13)
Acetohydroxyacid synthase (AHAS) is a thiamin diphosphate dependent enzyme that catalyses the decarboxylation of pyruvate to yield the hydroxyethyl-thiamin diphosphate (ThDP) anion/enamine intermediate (HEThDP(-)). This intermediate reacts with a second ketoacid to form acetolactate or acetohydroxybutyrate as products. Whereas the
C Monnet et al.
Letters in applied microbiology, 36(6), 399-405 (2003-05-20)
To demonstrate the presence of an active alpha-acetolactate decarboxylase in Streptococcus thermophilus and to investigate its physiological function. Streptococcus thermophilus CNRZ385 contains a gene encoding an alpha-acetolactate decarboxylase. Comparison of the production of alpha-acetolactate and its decarboxylation products, by the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service