W285609
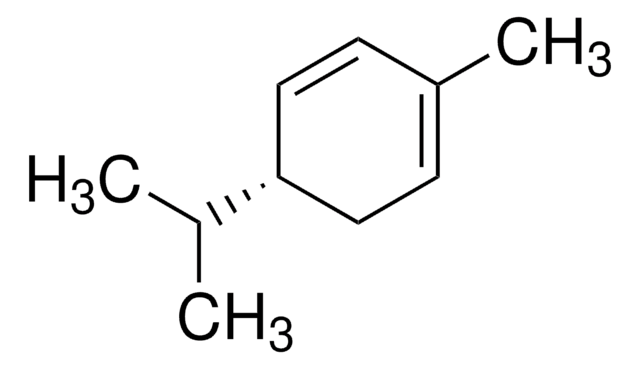
α-Phellandrene
≥75%, stabilized
Synonym(s):
2-Methyl-5-(1-methylethyl)-1,3-cyclohexadiene
About This Item
Recommended Products
biological source
synthetic
Quality Level
grade
Halal
Kosher
reg. compliance
FDA 21 CFR 172.515
Assay
≥75%
optical activity
[α]20/D −139 to −110°, neat
contains
alpha-tocopherol as additive
refractive index
n20/D 1.474 (lit.)
density
0.85 g/mL at 25 °C (lit.)
application(s)
flavors and fragrances
Documentation
see Safety & Documentation for available documents
food allergen
no known allergens
Organoleptic
spicy; woody
SMILES string
CC(C)C1CC=C(C)C=C1
InChI
1S/C10H16/c1-8(2)10-6-4-9(3)5-7-10/h4-6,8,10H,7H2,1-3H3
InChI key
OGLDWXZKYODSOB-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
- Does green mean clean Volatile organic emissions from regular versus green cleaning products.: This research compares the volatile organic compound (VOC) emissions from conventional and green cleaning products, identifying a-phellandrene as a common component. The findings highlight the environmental impact of green products and their effectiveness (Harding-Smith et al., 2024).
- RNA-Seq-Based Transcriptomics and GC-MS Quantitative Analysis Reveal Antifungal Mechanisms of Essential Oil of Clausena lansium (Lour.) Skeels Seeds against Candida albicans.: The study uses RNA-Seq and GC-MS to investigate the antifungal properties of Clausena lansium essential oil, which contains a-phellandrene, against Candida albicans, providing insights into its potential therapeutic applications (Ma et al., 2023).
- Unveiling the Anti-Cholera and Active Diabetic Renoprotective Compounds of Maqian Essential Oil: A Computational and Molecular Dynamics Study.: This study identifies a-phellandrene as an active compound in Maqian essential oil with anti-cholera and renoprotective effects, suggesting its potential for treating cholera and protecting against diabetic renal damage (Dahab et al., 2023).
- Electrophysiological and Behavioral Responses of Batocera horsfieldi Hope to Volatiles from Pistacia chinensis Bunge.: This research examines the responses of Batocera horsfieldi to volatiles, including a-phellandrene, from Pistacia chinensis. The findings have implications for pest management strategies using natural volatiles (Fan et al., 2023).
Disclaimer
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Asp. Tox. 1 - Flam. Liq. 3
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
116.6 °F - closed cup
Flash Point(C)
47 °C - closed cup
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Protocols
Fast GC analysis of sweet orange essential oil in hexane. Key components identified includes: β-Farnesene; α-Huµlene; Germacrene D; (+)-Valencene; Bicyclogermacrene; (+)-δ-Cadinene
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service