929484
FBnG-C3-PEG1-C3-NH2 hydrochloride
≥95%
Synonym(s):
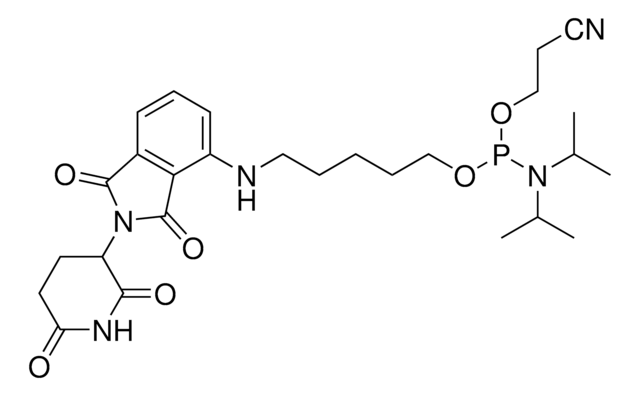
(R)-2-acetamido-3-((2-amino-9-(4-fluorobenzyl)-6-oxo-6,9-dihydro-1H-purin-8-yl)thio)-N-(3-(3-aminopropoxy)propyl)propanamide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C23H31FN8O4S · xHCl
Molecular Weight:
534.61 (free base basis)
UNSPSC Code:
12352101
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥95%
form
powder
functional group
amine
storage temp.
2-8°C
SMILES string
O=C1NC(N)=NC2=C1N=C(SC[C@@H](C(NCCCOCCCN)=O)NC(C)=O)N2CC3=CC=C(C=C3)F.Cl
Related Categories
Application
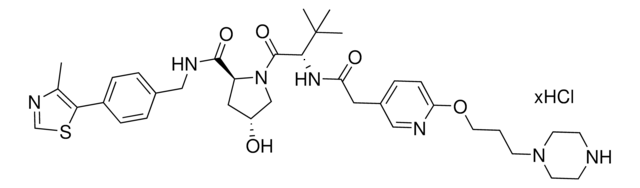
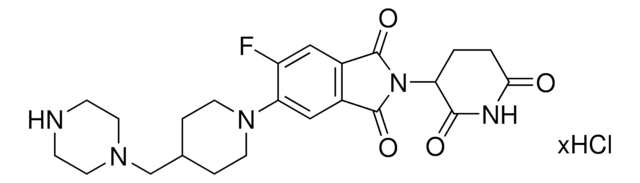
Protein degrader building block FBnG-C3-PEG1-C3-NH2 hydrochloride enables the synthesis of molecules for degradation of proteins and PROTAC® (proteolysis-targeting chimeras) research. This conjugate contains a p-fluorobenzylguanine (FBnG) ligand, a PEG linker, and a pendant amine for reactivity with a carboxylic acid on the target ligand. Because even slight alterations in ligands and crosslinkers can affect ternary complex formation between the target, E3 ligase, and degrader, many analogs are prepared to screen for optimal target degradation. When used with other protein degrader building blocks with a terminal amine, parallel synthesis can be used to more quickly generate degrader libraries that feature variation in crosslinker length, composition, and E3 ligase ligand.
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Technology Spotlight: Degrader Building Blocks for Targeted Protein Degradation
Protein Degrader Building Blocks
Other Notes
Legal Information
PROTAC is a registered trademark of Arvinas Operations, Inc., and is used under license
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Vittoria Zoppi et al.
Journal of medicinal chemistry, 62(2), 699-726 (2018-12-13)
Developing PROTACs to redirect the ubiquitination activity of E3 ligases and potently degrade a target protein within cells can be a lengthy and unpredictable process, and it remains unclear whether any combination of E3 and target might be productive for
Daiki Takahashi et al.
Molecular cell, 76(5), 797-810 (2019-10-14)
Protein silencing represents an essential tool in biomedical research. Targeted protein degradation (TPD) strategies exemplified by PROTACs are rapidly emerging as modalities in drug discovery. However, the scope of current TPD techniques is limited because many intracellular materials are not
Daniel P Bondeson et al.
Annual review of pharmacology and toxicology, 57, 107-123 (2016-10-13)
Protein homeostasis networks are highly regulated systems responsible for maintaining the health and productivity of cells. Whereas therapeutics have been developed to disrupt protein homeostasis, more recently identified techniques have been used to repurpose homeostatic networks to effect degradation of
Momar Toure et al.
Angewandte Chemie (International ed. in English), 55(6), 1966-1973 (2016-01-13)
The current inhibitor-based approach to therapeutics has inherent limitations owing to its occupancy-based model: 1) there is a need to maintain high systemic exposure to ensure sufficient in vivo inhibition, 2) high in vivo concentrations bring potential for off-target side effects, and 3) there is
Kedra Cyrus et al.
Molecular bioSystems, 7(2), 359-364 (2010-10-06)
Conventional genetic approaches have provided a powerful tool in the study of proteins. However, these techniques often preclude selective manipulation of temporal and spatial protein functions, which is crucial for the investigation of dynamic cellular processes. To overcome these limitations
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service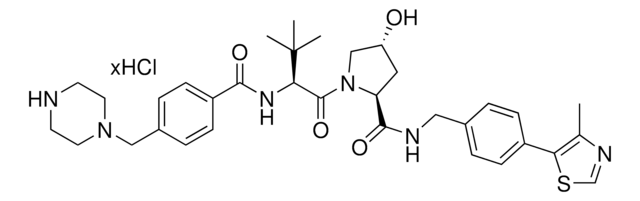
![Poly[(9,9-dioctyl-2,7-divinylenefluorenylene)-alt-{2-methoxy-5-(2-ethylhexyloxy)-1,4-phenylene}]](/deepweb/assets/sigmaaldrich/product/structures/303/311/48d96c0b-9463-4dd8-9ccf-3a67b7c781c5/640/48d96c0b-9463-4dd8-9ccf-3a67b7c781c5.png)



![1,4-Bis[4-(6-acryloyloxyhexyloxy)benzoyloxy]-2-methylbenzene ≥95%](/deepweb/assets/sigmaaldrich/product/structures/348/602/d338a771-03b4-4ba9-9788-426be85146d9/640/d338a771-03b4-4ba9-9788-426be85146d9.png)