523976
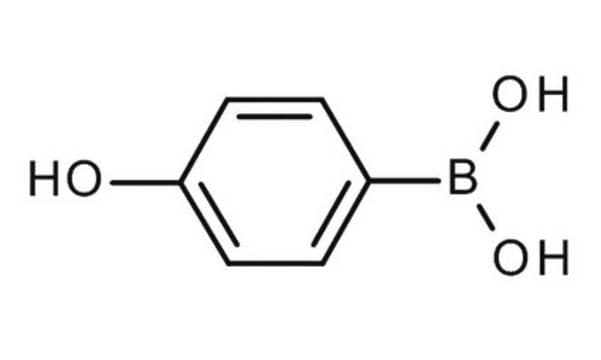
4-Hydroxyphenylboronic acid
≥95.0%
Synonym(s):
(p-Hydroxyphenyl)boronic acid, 4-Hydroxybenzeneboronic acid, p-hydroxy-benzeneboronic acid
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
HOC6H4B(OH)2
CAS Number:
Molecular Weight:
137.93
MDL number:
UNSPSC Code:
12352103
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥95.0%
form
solid
mp
>230 °C (lit.)
SMILES string
OB(O)c1ccc(O)cc1
InChI
1S/C6H7BO3/c8-6-3-1-5(2-4-6)7(9)10/h1-4,8-10H
InChI key
COIQUVGFTILYGA-UHFFFAOYSA-N
Related Categories
Application
4-Hydroxyphenylboronic acid can be used as a reactant in:
It can also be used to prepare/promote:
- Suzuki-Miyaura coupling and Stille coupling reactions.
- Palladium-catalyzed aminocarbonylation and cross-coupling reactions.
- Suzuki reaction for preparation of bio-supported palladium nanoparticles as phosphine-free catalysts.
- Cu2O-catalyzed aerobic oxidative cross-coupling of tetrazoles.
It can also be used to prepare/promote:
- PDK1 inhibitory activity (cancer cell growth, survival, and tumorigenesis inhibitor).
- Rod-like dendronized polymers containing G4 and G5 ester dendrons via macromonomer approach by living ROMP.
- Estrone-derived cyclopamine analogs as Sonic Hedgehog signaling inhibitors for anti-cancer chemotherapeutics.
- Enzymatic inhibitors for the treatment of Gram-negative bacterial infections.
- Oligoarenes by Suzuki-Miyaura palladium-catalyzed cross-coupling.
Other Notes
Contains varying amounts of anhydride
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Tetrahedron Letters, 48, 845-845 (2007)
Scott E Denmark et al.
Journal of the American Chemical Society, 128(50), 15958-15959 (2006-12-15)
The cross-coupling of geometrically defined (E)- and (Z)-alkenyl- and styrylsilanolates with a wide variety of aromatic and heteroaromatic chlorides has been achieved. Under catalysis by bulky, biphenyl-derived phosphines and allylpalladium chloride, the (preformed, stable) potassium salts of di-, tri- and
Ishikawa, Shunpei; Manabe, Kei
Chemistry Letters (Jpn), 35, 164-165 (2006)
Highly selective palladium-catalyzed aminocarbonylation and cross-coupling reactions on a cavitand scaffold
Csok, Z.; Takatsy, A.; Kollar, L.
Tetrahedron, 68, 2657-2661 (2012)
Synthetic approach to the chemical isostere of O-methyl honokiol
Cui, M.; Kim, H. S.
Synlett, 23, 311-313 (2012)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service