444286
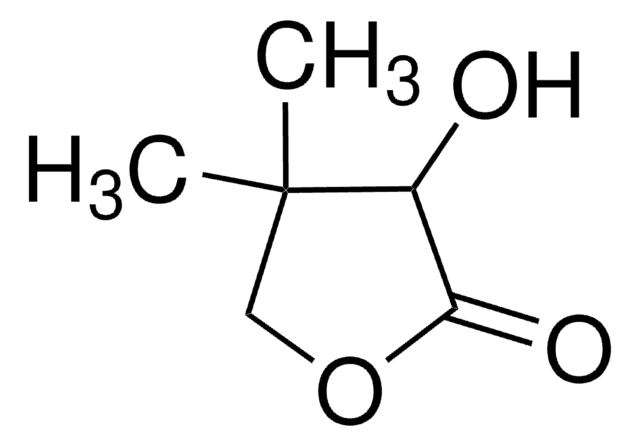
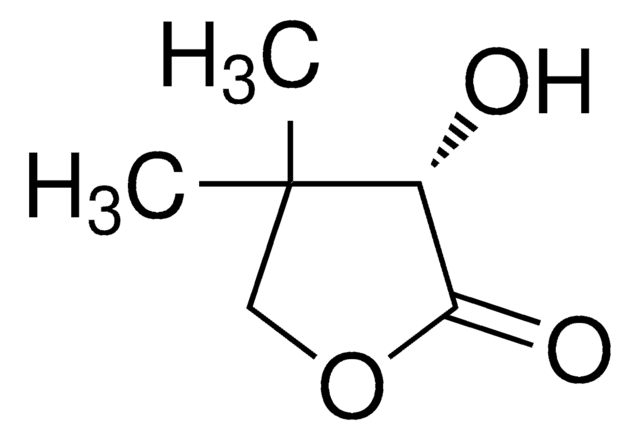
(R)-(+)-α-Hydroxy-γ-butyrolactone
95%, optical purity ee: 98% (GLC)
Synonym(s):
(R)-4,5-Dihydro-3-hydroxy-2(3H)-furanone
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C4H6O3
CAS Number:
Molecular Weight:
102.09
Beilstein:
80588
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
optical activity
[α]23/D +66°, c = 1.15 in chloroform
optical purity
ee: 98% (GLC)
refractive index
n20/D 1.467 (lit.)
bp
133 °C/10 mmHg (lit.)
density
1.309 g/mL at 25 °C (lit.)
functional group
ester
hydroxyl
SMILES string
O[C@@H]1CCOC1=O
InChI
1S/C4H6O3/c5-3-1-2-7-4(3)6/h3,5H,1-2H2/t3-/m1/s1
InChI key
FWIBCWKHNZBDLS-GSVOUGTGSA-N
Application
(R)-(+)-α-Hydroxy-γ-butyrolactone can be used as a starting material to synthesize:
- δ-Azaproline by reacting with benzyloxycarbonyl aminophthalimide via Mitsunobu reactions.
- Homochiral (R)-2,4-dihydroxybutyramide seco-pseudonucleoside reagents.
- Botryolide B via esterification and ring-closing metathesis reaction.
- Pregnane derivatives containing γ-butyrolactones as potential glucocorticoid agonists.
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Novel glucocorticoid antedrugs possessing a 21-(?-lactone) ring.
Angell RM, et al.
Journal of the Chemical Society. Perkin Transactions 1, 6, 831-839 (2002)
Concise total synthesis of botryolide B
Mohapatra DK, et al.
Royal Society of Chemistry Advances, 4(16), 8335-8340 (2014)
Novel glucocorticoid antedrugs possessing a 21-(γ-lactone) ring
Angell RM, et al.
Journal of the Chemical Society. Perkin Transactions 1, 4(6), 831-839 (2002)
Xiaohui Gou et al.
Frontiers in physiology, 11, 686-686 (2020-07-17)
Dentin sialoprotein (DSP), the NH2-terminal fragment of dentin sialophosphoprotein (DSPP), is essential for dentin formation and further processed into small fragments inside the odontoblasts. Gelatinases, including matrix metalloproteinases 9 (MMP9) and MMP2, were able to cleave DSP(P) in tooth structures.
Efficient synthesis of enantiomerically pure (S)-d-azaproline starting from (R)-a-hydroxy-?-butyrolactone via the Mitsunobu reaction.
Voss E, et al.
Tetrahedron Asymmetry, 20(15), 1809-1812 (2009)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service