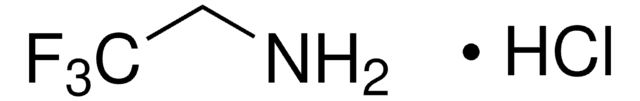
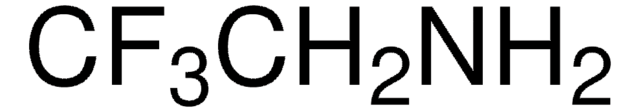429058
2-Fluoroethylamine hydrochloride
technical grade, 90%
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Linear Formula:
FCH2CH2NH2 · HCl
CAS Number:
Molecular Weight:
99.54
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
90%
form
solid
mp
99-103 °C (lit.)
functional group
amine
fluoro
SMILES string
Cl.NCCF
InChI
1S/C2H6FN.ClH/c3-1-2-4;/h1-2,4H2;1H
InChI key
YRRZGBOZBIVMJT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
2-Fluoroethylamine hydrochloride is a fluorinated alkylammonium salt. Studies suggest that adding a fluorine atom to it leads to double gauche effect in the resulting 2,2-difluoroethylamine chloride. Microwave spectral studies for 2-fluoroethylamine has been conducted.
Application
2-Fluoroethylamine hydrochloride may be used in the synthesis of the following:
- 2-fluoroethylisocyanate
- 1,3-bis-(2-fluoroethyl) urea (BFU)
- N-2-fluoroethylmaleimide
- [Cu4(O)(Ln)2(CH3COO)4] where HL= 4-methyl-2,6-bis(2-fluoroethyliminomethyl) phenol
- 1-O-(4-(2-fluoroethyl-carbamoyloxymethyl)-2-nitrophenyl)-O-β-D-glucopyronuronate (FEAnGA)
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Microwave-spectrum, conformational equilibrium, intramolecular hydrogen-bonding, dipole-moments, N-14 nuclear-quadrupole coupling-constants and centrifugal-distortion constants of 2-fluoroethylamine.
Marstokk KM and Mollendal H.
Acta Chemica Scandinavica, 34(1), 15-29 (1980)
Tetranuclear copper (II)-Schiff-base complexes as active catalysts for oxidation of cyclohexane and toluene.
Roy P and Manassero M.
Dalton Transactions, 39(6), 1539-1545 (2010)
Synthesis of 18F-labelled 2-fluoroethyl-nitrosoureas.
Farrokhzad S, et al.
Canadian Journal of Chemistry, 62(11), 2107-2112 (1984)
Conformational analysis of 2, 2-difluoroethylamine hydrochloride: double gauche effect.
Silla JM, et al.
Beilstein Journal of Organic Chemistry, 10(1), 877-882 (2014)
Development of a new thiol site-specific prosthetic group and its conjugation with [Cys40]-exendin-4 for in vivo targeting of insulinomas.
Yue X, et al.
Bioconjugate Chemistry, 24(7), 1191-1200 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service