384305
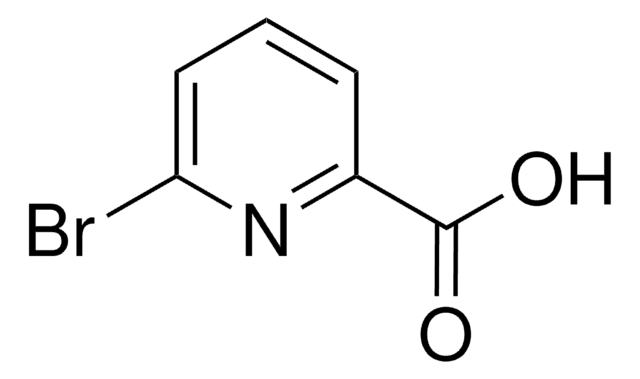
6-Hydroxypyridine-2-carboxylic acid
95%
Synonym(s):
6-Hydroxypicolinic acid, 6-Hydroxypyridine-2-carboxylic acid
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C6H5NO3
CAS Number:
Molecular Weight:
139.11
Beilstein:
115842
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
solid
mp
270 °C (dec.) (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)c1cccc(O)n1
InChI
1S/C6H5NO3/c8-5-3-1-2-4(7-5)6(9)10/h1-3H,(H,7,8)(H,9,10)
InChI key
VRCWSYYXUCKEED-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
6-Hydroxypyridine-2-carboxylic acid (6HPA, 6-Hydroxypicolinic acid) is a picolinic acid derivative. It has been reported to exhibit enol-keto tautomerism. It is a chelating ligand exhibiting potential complexing ability (via N,O-chelation or N,O,O-chelation). Intramolecular proton transfer (IPT) in tautomeric forms of 6HPA has been investigated by density functional theory (DFT) calculations.
Application
6-Hydroxypyridine-2-carboxylic acid may be used in the preparation of ruthenium(II) complex, [RuH(CO)(6-OH-py-2-COO)(PPh3)2].
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Computational study of the intramolecular proton transfer between 6-hydroxypicolinic acid tautomeric forms and intermolecular hydrogen bonding in their dimers.
Kazemi Riabi SH, et al.
Physical Chemistry Research, 1, 117-125 (2013)
Synthesis, molecular, spectroscopic and catalytic characterization of ruthenium (II) complexes with pyridine-2-carboxylic acid derivatives ligands.
Malecki JG, et al.
Polyhedron, 48(1), 21-30 (2012)
Jennifer A Jacobsen et al.
Journal of medicinal chemistry, 54(2), 591-602 (2010-12-30)
Fragment-based lead design (FBLD) has been used to identify new metal-binding groups for metalloenzyme inhibitors. When screened at 1 mM, a chelator fragment library (CFL-1.1) of 96 compounds produced hit rates ranging from 29% to 43% for five matrix metalloproteases
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service