All Photos(1)
About This Item
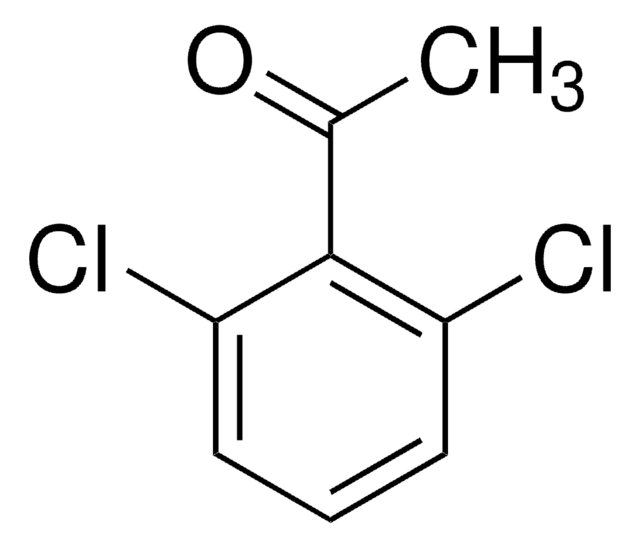
Linear Formula:
Cl2C6H3COCH3
CAS Number:
Molecular Weight:
189.04
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
96%
form
solid
refractive index
n20/D 1.5635 (lit.)
bp
140-150 °C/15 mmHg (lit.)
mp
33-34 °C (lit.)
functional group
chloro
ketone
SMILES string
CC(=O)c1ccc(Cl)cc1Cl
InChI
1S/C8H6Cl2O/c1-5(11)7-3-2-6(9)4-8(7)10/h2-4H,1H3
InChI key
XMCRWEBERCXJCH-UHFFFAOYSA-N
Related Categories
Application
2′,4′-Dichloroacetophenone was used in the synthesis of the Schiff base derivatives.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Dam. 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 2
Flash Point(F)
235.4 °F - closed cup
Flash Point(C)
113 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Xue-yan Shi et al.
Se pu = Chinese journal of chromatography, 20(1), 34-36 (2003-01-25)
A new cyclodextrin (CD) derivative, 2,6-di-O-benzyl-3-O-valeryl-beta-CD, was synthesized and characterized by 1H NMR and IR. Using the beta-CD derivative as chiral stationary phase of capillary gas chromatography, one chiral column was prepared. On this column, the enantiomeric excesses (e.e.) of
Microwave assisted rapid and efficient synthesis of nitrogen and sulphur containing heterocyclic compounds and their pharmacological evaluation.
Indian J. Chem. B, 45(7), 1762-1762 (2006)
A Brundin et al.
Biochemical pharmacology, 31(23), 3885-3890 (1982-12-01)
alpha-Haloketones are highly reactive compounds, which are known to undergo enzymatic reduction to methyl ketones. The objective of this research was to characterize the enzymes involved in this reaction and to investigate the mechanism of the reaction. 2,2',4'-Trichloroacetophenone was reduced
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service