139521
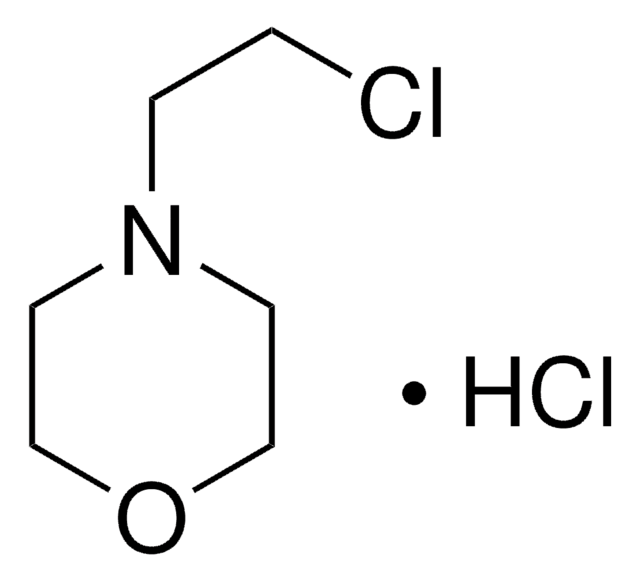
2-(2-Chloroethyl)-1-methylpyrrolidine hydrochloride
99%
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C7H14ClN · HCl
CAS Number:
Molecular Weight:
184.11
Beilstein:
6148386
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
mp
98-102 °C (lit.)
functional group
chloro
SMILES string
Cl[H].CN1CCCC1CCCl
InChI
1S/C7H14ClN.ClH/c1-9-6-2-3-7(9)4-5-8;/h7H,2-6H2,1H3;1H
InChI key
KCQMALZNENFGKK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
2-(2-Chloroethyl)-1-methylpyrrolidine hydrochloride was used in the synthesis of N-[2-(1-methylpyrrolidin-2-yl)ethyl]-N-(2-iodo-4,5-dimethoxyphenyl) amide. It was used as reagent during the synthesis of 1-methyl-2-[2-[2-(2-phenylethyl)phenoxy]ethyl]pyrrolidine hydrochlochloride.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
N Tanaka et al.
Chemical & pharmaceutical bulletin, 48(2), 245-255 (2000-03-08)
A series of [2-(omega-phenylalkyl)phenoxy]alkylamines was synthesized and their receptor binding affinity was examined in vitro. These compounds showed an affinity for serotonin-2 (5-HT2) and dopamine-2 (D2) receptors. [2-(2-phenylethyl)phenoxy]alkylamine derivatives with a pyrrolidine or piperidine moiety in the structure showed higher
Shejin Zhu et al.
Bioorganic & medicinal chemistry, 14(9), 3131-3143 (2006-01-18)
6-Substituted 8,9-dimethoxy-2,3-methylenedioxy-6H-dibenzo[c,h][2,6]naphthyridin-5-ones were synthesized and evaluated for topoisomerase I-targeting activity and cytotoxicity. Several of these reversed lactam analogues of ARC-111 exhibited exceptional cytotoxicity with IC50 values ranging from 0.5 to 3.0 nM. In contrast to topotecan, no resistance was observed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service