Q2763
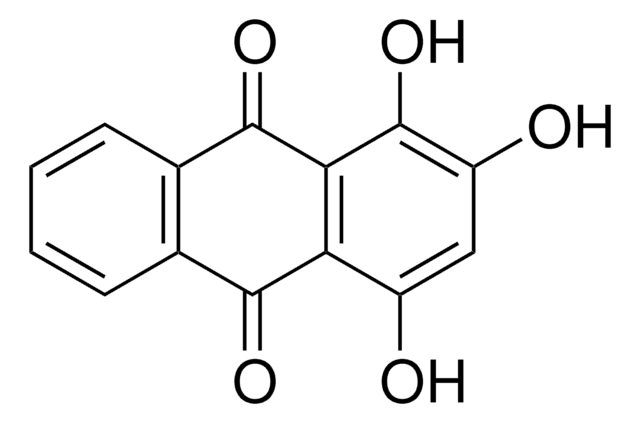
Quinalizarin
≥95% (HPLC), powder, plasmodium falciparum casein kinase 2α (PfCK2α) inhibitor
Synonym(s):
1,2,5,8-Tetrahydroxy-9,10-anthraquinone, 1,2,5,8-Tetrahydroxyanthraquinone, Alizarin Bordeaux BD, Alizarinbordeaux, Alizarine Bordeaux, Alizarine Bordeaux B, C.I. 58500, C.I. Mordant Violet 26, Khinalizarin, NSC 144046, NSC 4896, PHF 016
About This Item
Recommended Products
product name
Quinalizarin, ≥95% (HPLC)
Assay
≥95% (HPLC)
form
powder
color
red
mp
≥300 °C
storage temp.
room temp
SMILES string
Oc1ccc2C(=O)c3c(O)ccc(O)c3C(=O)c2c1O
InChI
1S/C14H8O6/c15-6-3-4-7(16)11-10(6)12(18)5-1-2-8(17)13(19)9(5)14(11)20/h1-4,15-17,19H
InChI key
VBHKTXLEJZIDJF-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- as a casein kinase II (CKII) inhibitor to study its effect on CKII-mediated phosphorylation of importin α on subcellular scaling in sperm chromosomes and egg extract
- as a CKII inhibitor to study its ability to block parasite multiplication in a [3H]-hypoxanthine incorporation assay
- to study its effect on colorectal cancer (CRC) cell cycle arrest, cell apoptosis, and reactive oxygen species (ROS) generation in SW480 and HCT-116 cell lines
Biochem/physiol Actions
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Aquatic Acute 1 - Aquatic Chronic 1
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service