G6104
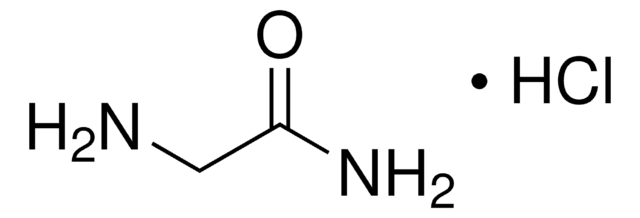
Glycinamide hydrochloride
98%
Synonym(s):
2-Aminoacetamide hydrochloride, Aminoacetamide hydrochloride, Glycine amide hydrochloride
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
NH2CH2CONH2 · HCl
CAS Number:
Molecular Weight:
110.54
Beilstein:
3554199
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
98%
mp
204 °C (dec.) (lit.)
SMILES string
Cl.NCC(N)=O
InChI
1S/C2H6N2O.ClH/c3-1-2(4)5;/h1,3H2,(H2,4,5);1H
InChI key
WKNMKGVLOWGGOU-UHFFFAOYSA-N
Application
Buffer useful in the physiological pH range.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Gottfried K Schroeder et al.
Biochemistry, 46(13), 4037-4044 (2007-03-14)
As a model for mechanistic comparison with peptidyl transfer within the ribosome, the reaction of aqueous glycinamide with N-formylphenylalanine trifluoroethyl ester (fPhe-TFE) represents an improvement over earlier model reactions involving Tris. The acidity of trifluoroethanol (pKa 12.4) resembles that of
Eric Loeser et al.
Analytical chemistry, 79(14), 5382-5391 (2007-05-29)
When mobile-phase salt content is increased, cationic analytes often show increased retention. This effect is generally attributed to chaotropic or ion pairing effects. However, a cation exclusion mechanism could explain the same effects. In this study, experimental conditions were manipulated
Brett C Bookser et al.
Journal of medicinal chemistry, 48(24), 7808-7820 (2005-11-24)
4-(Phenylamino)-5-phenyl-7-(5-deoxy-beta-D-ribofuranosyl)pyrrolo[2,3-d]pyrimidine 1 and related compounds known as "diaryltubercidin" analogues are potent inhibitors of adenosine kinase (AK) and are orally active in animal models of pain such as the rat formalin paw model (GP3269 ED50= 6.4 mg/kg). However, the utility of
Xiaodong Jia et al.
The Journal of organic chemistry, 78(18), 9450-9456 (2013-08-21)
A catalytic α-sp(3) C-H oxidation of peptides and glycine amides was achieved under radical cation salt catalysis in the presence of O2, producing a series of substituted quinolines. The scope of this reaction shows good functional group tolerance and high
Len Ito et al.
FEBS letters, 585(3), 555-560 (2011-01-18)
Glycine amide (GlyAd), a typically amidated amino acid, is a versatile additive that suppresses protein aggregation during refolding, heat treatment, and crystallization. In spite of its effectiveness, the exact mechanism by which GlyAd suppresses protein aggregation remains to be elucidated.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service