All Photos(1)
About This Item
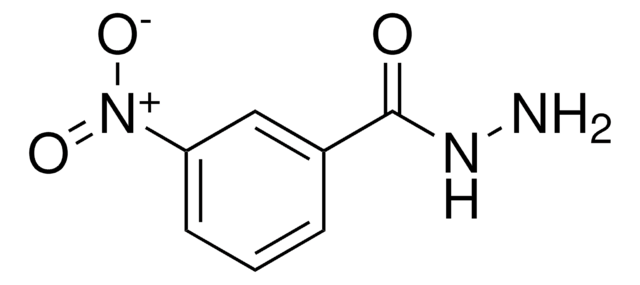
Linear Formula:
H2NC6H4C(O)NHNH2
CAS Number:
Molecular Weight:
151.17
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
95%
form
powder
mp
225-227 °C (lit.)
SMILES string
NNC(=O)c1ccc(N)cc1
InChI
1S/C7H9N3O/c8-6-3-1-5(2-4-6)7(11)10-9/h1-4H,8-9H2,(H,10,11)
InChI key
WPBZMCGPFHZRHJ-UHFFFAOYSA-N
Application
4-Aminobenzoic hydrazide can be used as a reactant to synthesize:
- Acylhydrazone Schiff base ligand by condensation reaction with 2-acetylpyridine.
- Oligomeric transition metal complexes applicable in the fabrication of organic-inorganic hybrid devices with efficient electrical and photoelectrical properties.
- Oxovanadium(IV)-hydrazide complex as a potential radical scavenger.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Preparation of porous resin with Schiff base chelating groups for removal of heavy metal ions from aqueous solutions
Ceglowski M and Schroeder G
Chemical Engineering Journal, 263 (2015)
U Burner et al.
The Journal of biological chemistry, 274(14), 9494-9502 (1999-03-27)
Myeloperoxidase is the most abundant protein in neutrophils and catalyzes the production of hypochlorous acid. This potent oxidant plays a central role in microbial killing and inflammatory tissue damage. 4-Aminobenzoic acid hydrazide (ABAH) is a mechanism-based inhibitor of myeloperoxidase that
G O Peelen et al.
Analytical biochemistry, 198(2), 334-341 (1991-11-01)
Analysis of oligosaccharides in complex biological matrices is hampered by the fact that oligosaccharides, closely related in structure, are difficult to separate from each other and that conventional detection procedures (refraction index and uv detection) are not specific enough for
A J Kettle et al.
The Biochemical journal, 308 ( Pt 2), 559-563 (1995-06-01)
Myeloperoxidase is the most abundant protein in neutrophils and catalyses the conversion of H2O2 and chloride into HOCl. To help clarify the role of this enzyme in bacterial killing and inflammation, a specific and potent inhibitor needs to be identified.
Electrical and photoelectrical behaviour of heterojunctions based on novel oligomeric metal complexes
Atlan M, et al.
Applied Organometallic Chemistry, 29(12) (2015)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service