383473
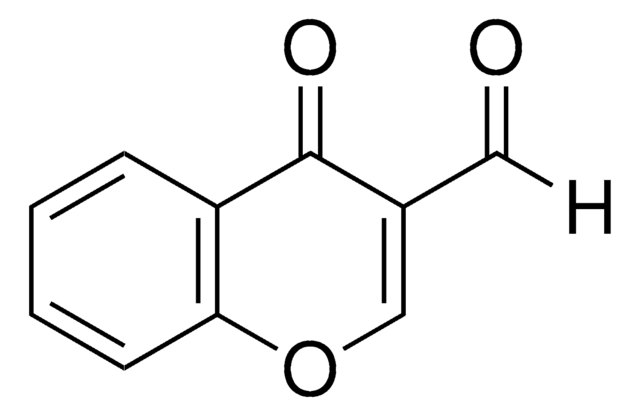
3-Formyl-6-methylchromone
97%
Synonym(s):
6-Methyl-4-oxo-4H-1-benzopyran-3-carboxaldehyde
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C11H8O3
CAS Number:
Molecular Weight:
188.18
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
172-173 °C (lit.)
SMILES string
Cc1ccc2OC=C(C=O)C(=O)c2c1
InChI
1S/C11H8O3/c1-7-2-3-10-9(4-7)11(13)8(5-12)6-14-10/h2-6H,1H3
InChI key
GBWMIOYSMWCYIZ-UHFFFAOYSA-N
General description
3-Formyl-6-methylchromone is a 3-formyl-substituted chromone. The combustion calorimetric estimation of the enthalpy of combustion of 3-formyl-6-methylchromone has been reported. Its anti-proliferative action on the MDR human colon cancer and mouse lymphoma has been investigated.
Application
3-Formyl-6-methylchromone has been used for the synthesis of 3-formyl-6-methylchromone-4-phenylthiosemicarbazone.
3-Formyl-6-methylchromone may be used in the preparation of series of Schiff′s bases, via reaction with aromatic sulfonamides, such as sulfanilamide, homosulfanilamide, 4-aminoethyl-benzenesulfonamide, a pyrimidinyl-substituted sulfanilamide derivative, sulfaguanidine and 4-amino-6-trifluoromethyl-benzene-1,3-disulfonamide. It may be used in the preparation of Schiff base, which undergoes addition reaction with diethyl phosphite to afford bis[(1,2,4,3-triazaphospholyl)(chromonyl)aminomethylphosphonate] derivative and bis[(1,2,4,3-triazaphospholyl)(chromonyl)phosphorylamino-methylphosphonate] derivative.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthetic approach for novel bis (a-aminophosphonic acid) derivatives of chromone containing 1, 2, 4, 3-triazaphosphole moieties
Ali, TE and Halacheva, SS.
Heteroatom Chem., 20(3), 117-122 (2009)
Luca Puccetti et al.
Bioorganic & medicinal chemistry letters, 15(12), 3096-3101 (2005-05-24)
A series of Schiff's bases was prepared by reaction of 3-formyl-chromone or 6-methyl-3-formyl-chromone with aromatic sulfonamides, such as sulfanilamide, homosulfanilamide, 4-aminoethyl-benzenesulfonamide, a pyrimidinyl-substituted sulfanilamide derivative, sulfaguanidine and 4-amino-6-trifluoromethyl-benzene-1,3-disulfonamide. The zinc complexes of these sulfonamides have also been obtained. The new
Synthesis, characterization, crystal structure and antimicrobial activity of copper (II) complexes with a thiosemicarbazone derived from 3-formyl-6-methylchromone.
Ilies DC, et al.
Polyhedron, 81, 123-131 (2014)
Enthalpies of combustion and formation of 3-formylchromones.
Flores H, et al.
Thermochimica Acta, 450(1), 35-37 (2006)
Farukh Arjmand et al.
Bioorganic chemistry, 104, 104327-104327 (2020-11-05)
Copper-based antitumor drug entities 1-3 derived from substituted (F-, Br-, -CH3) 3-formylchromone pharmacophore were synthesized and thoroughly characterized by spectroscopic and single X-ray crystallographic studies. These complexes show structural novelty due to presence of the X-bonds in chromone scaffold which
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service