All Photos(1)
About This Item
Empirical Formula (Hill Notation):
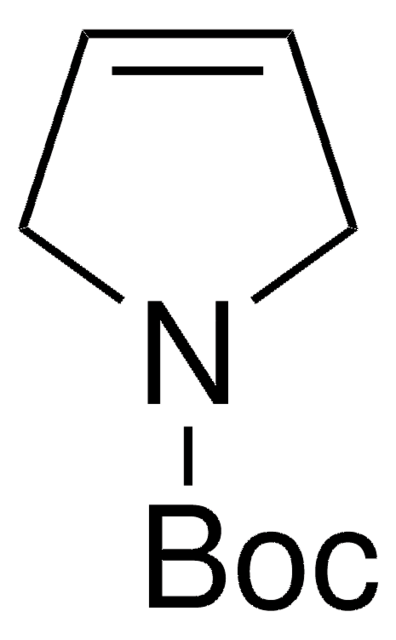
C4H7N
CAS Number:
Molecular Weight:
69.11
Beilstein:
103173
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
95%
form
liquid
refractive index
n20/D 1.46 (lit.)
bp
90-91 °C/748 mmHg (lit.)
density
0.91 g/mL at 25 °C (lit.)
storage temp.
−20°C
SMILES string
C1NCC=C1
InChI
1S/C4H7N/c1-2-4-5-3-1/h1-2,5H,3-4H2
InChI key
JVQIKJMSUIMUDI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
3-Pyrroline is a heterocyclic building block. The excited state dynamics of 3-pyrroline by Hamiltonian model based on the vibronic coupling model has been investigated. Trifluoromethylated azomethine ylide is reported as precursor for the synthesis of 3-pyrroline building blocks. 3-Pyrrolines are reported as highly useful intermediates for the synthesis of functionalized pyrrolines, pyrrolidines and other natural products. Preparation of 3-pyrroline(2,5-dihydro-1H-pyrrole) from (Z)-1,4-dichloro-2-butene, via Delépine Reaction has been reported. It is formed as intermediate in the synthesis of N-(tert-butyloxycarbonyl)-3-pyrroline. Reaction of Me3Al and Me3Ga with 3-pyrroline is reported.
Various 3-pyrrolines (2,5-dihydropyrroles) have been synthesized by two-step reaction sequence of alkylation/alkylidene carbene CH-insertion reaction. Synthesis of 3-pyrroline has been reported by employing cis-1,4-dichloro-2-butene as starting reagent.
Application
3-Pyrroline is suitable for use in a study to investigate the core-level binding energies of simple unsaturated organic molecules bonded to the Si(001) surface by X-ray photoelectron spectroscopy (XPS). It may be used in the synthesis of renin inhibitors and vasodilators.
accessory
Product No.
Description
Pricing
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Inhalation - Acute Tox. 4 Oral - Flam. Liq. 2 - Skin Corr. 1A
Storage Class Code
3 - Flammable liquids
WGK
WGK 2
Flash Point(F)
-0.4 °F - closed cup
Flash Point(C)
-18 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
S H Rosenberg et al.
Journal of medicinal chemistry, 33(7), 1962-1969 (1990-07-01)
Incorporation of nonreactive polar functionalities at the C- and N-termini of renin inhibitors led to the development of a subnanomolar compound (21) with millimolar solubility. This inhibitor demonstrated excellent efficacy and a long duration of action upon intravenous administration to
A modified procedure for the preparation of 2, 5-dihydropyrrole (3-pyrroline).
Warmus JS, et al.
The Journal of Organic Chemistry, 58(1), 270-271 (1993)
S P Neville et al.
The journal of physical chemistry. A, 118(51), 11975-11986 (2014-09-16)
A model Hamiltonian based on the vibronic coupling model is developed to describe the excited state dynamics of 3-pyrroline. With the use of the method of improved relaxation in conjunction with the MCTDH wavepacket propagation algorithm, vibrational eigenstates corresponding to
A convenient route to 3-pyrroline utilizing the Delepine reaction.
Brandange S and Rodriguez B.
Synthesis, 4 , 347-348 (1988)
An X-ray photoelectron spectroscopy study of the bonding of unsaturated organic molecules to the Si (001) surface.
Liu H and HamersRJ.
Surface Science, 416(3), 354-362 (1998)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service