349801
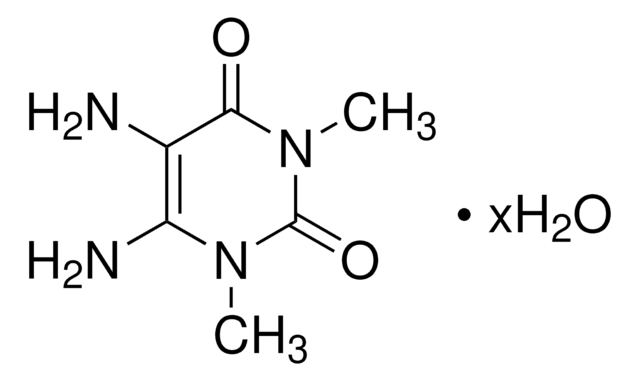
1,3-Dimethyluracil
99%
Synonym(s):
1,3-Dimethyl-2,4(1H,3H)-pyrimidinedione, 2,4-Dihydroxy-1,3-dimethylpyrimidine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C6H8N2O2
CAS Number:
Molecular Weight:
140.14
Beilstein:
124074
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
99%
form
powder
mp
119-122 °C (lit.)
SMILES string
CN1C=CC(=O)N(C)C1=O
InChI
1S/C6H8N2O2/c1-7-4-3-5(9)8(2)6(7)10/h3-4H,1-2H3
InChI key
JSDBKAHWADVXFU-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
1,3-Dimethyluracil is a pyrimidine derivative. Stability of the C6-centered carbanions derived from 1,3-dimethyluracil has been investigated in the gas phase and in DMSO and water solutions. The excited state structural dynamics of 1,3-dimethyluracil (DMU) in water and acetonitrile has been studied by resonance Raman spectroscopy. Crystal structure of 1,3-dimethyluracil has been reported. Ultraviolet irradiation of aqueous 1,3-dimethyluracil results in hydration of the 5:6 double bond of the uracil ring to form 1,3-dimethyl-6-oxy-hydrouracil.
Application
1,3-Dimethyluracil is suitable reagent used to investigate the steady-state absorption and fluorescence spectra of uracil derivatives. It may be used in the preparation of 2,6-dihydroxynicotinamide.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Amir Golan et al.
Journal of visualized experiments : JoVE, (68)(68), doi:10-doi:10 (2012-11-15)
Tunable soft ionization coupled to mass spectroscopy is a powerful method to investigate isolated molecules, complexes and clusters and their spectroscopy and dynamics(1-4). Fundamental studies of photoionization processes of biomolecules provide information about the electronic structure of these systems. Furthermore
[Thermal characteristics of the C--H...O hydrogen bonds formed by nucleic acid base analogs].
V I Bruskov et al.
Doklady Akademii nauk SSSR, 277(6), 1482-1486 (1984-01-01)
1, 3-Dimethyluracil: a crystal structure without hydrogen bonds.
Banerjee A, et al.
Acta Crystallographica Section B, Structural Science, 33(1), 90-94 (1977)
P F Heelis et al.
Photochemistry and photobiology, 57(3), 442-446 (1993-03-01)
Photosensitized splitting of cis-syn- and trans-syn-1,3-dimethyluracil dimers by 2',3',4',5'-tetraacetylriboflavin in acetonitrile containing a trace of perchloric acid was studied by laser flash photolysis. Protonation of the flavin prior to excitation resulted in excited singlet and triplet states that abstracted an
Singlet excited state dynamics of uracil and thymine derivatives: A femtosecond fluorescence upconversion study in acetonitrile.
Gustavsson T, et al.
Chemical Physics Letters, 429(4), 551-557 (2006)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service