252832
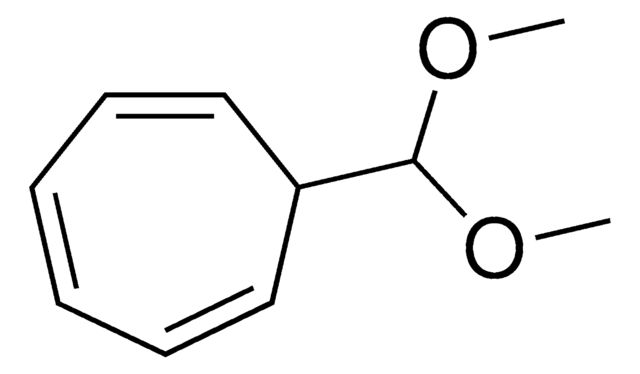
Tropone
97%
Synonym(s):
2,4,6-Cycloheptatrien-1-one
Sign Into View Organizational & Contract Pricing
All Photos(2)
About This Item
Empirical Formula (Hill Notation):
C7H6O
CAS Number:
Molecular Weight:
106.12
Beilstein:
1902335
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
liquid
refractive index
n20/D 1.615 (lit.)
bp
113 °C/15 mmHg (lit.)
density
1.094 g/mL at 25 °C (lit.)
functional group
ketone
storage temp.
−20°C
SMILES string
O=C1C=CC=CC=C1
InChI
1S/C7H6O/c8-7-5-3-1-2-4-6-7/h1-6H
InChI key
QVWDCTQRORVHHT-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
General description
Metal-catalyzed [6+3] cycloaddition of tropone with azomethine ylides has been reported.
Application
Tropone has been used in synthesis of:
- bicyclic δ-lactones via heterocyclic carbine-catalyzed [8+3] annulation pathway
- 6,7-benzobicyclo [3.2.2] nona-3,6,8-trien-2-one via thermal addition to bezyne
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
M Carmen Carreño et al.
Chemical communications (Cambridge, England), (8)(8), 1007-1009 (2005-02-19)
Synthesis of 4-aminotropones through a cyclization-ring expansion process occurs in a single step and with excellent yields from 4-amino-2,5-cyclohexadienones (p-quinamines) bearing a 4-sulfinyl or sulfonyl methyl group.
Yuji Matsuya et al.
Organic letters, 11(6), 1361-1364 (2009-02-24)
Efficient synthesis of 3,4-diazabenzo[b]tropone was first achieved utilizing 4pi-8pi sequential electrocyclic reactions of functionalized benzocyclobutenone derivatives. These compounds are highly electron deficient and readily form amine adducts at ambient temperature. Furthermore, gentle heating resulted in quantitative nitrogen extrusion to produce
Vijay Nair et al.
The Journal of organic chemistry, 71(23), 8964-8965 (2006-11-04)
A novel protocol for the annulation of tropone to enals involving nucleophilic heterocyclic carbene (NHC) catalyzed homoenolate formation has been developed. Interestingly, the reaction led to bicyclic delta-lactones instead of the expected gamma-spirolactones, presumably by the uncommon [8 + 3]
Barry M Trost et al.
Journal of the American Chemical Society, 130(45), 14960-14961 (2008-10-22)
The cyanosubstituted trimethylenemethane donor undergoes palladium-catalyzed [6 + 3] cycloaddition with a variety of tropones to yield bicyclo[4.3.1]decadienes in excellent regio-, diastereo-, and enantioselectivity. Products of the Pd-TMM [6 + 3] cycloaddition participate in a thermal [3,3] sigmatropic rearrangement to
M Carmen Carreño et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 14(2), 621-636 (2007-10-09)
An efficient synthesis of 4-aminotropones has been achieved in excellent yields by simple treatment of 4-amino-4-[(p-tolylsulfinyl)methyl]-2,5-cyclohexadienones (p-quinamines) with NaH. The method allowed regiocontrolled access to 3-methyl, 5-methyl- and 3,5-dimethyl-substituted derivatives starting from p-quinamines with adequate substituents at the cyclohexadienone moiety
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service