156361
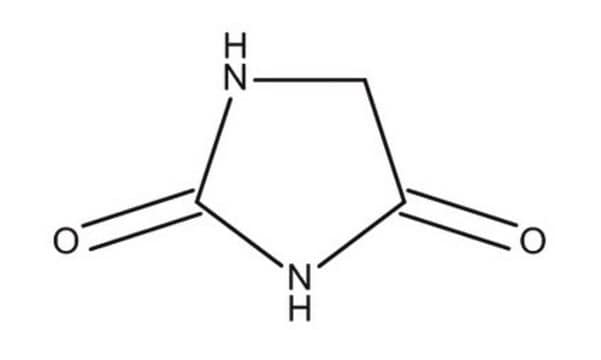
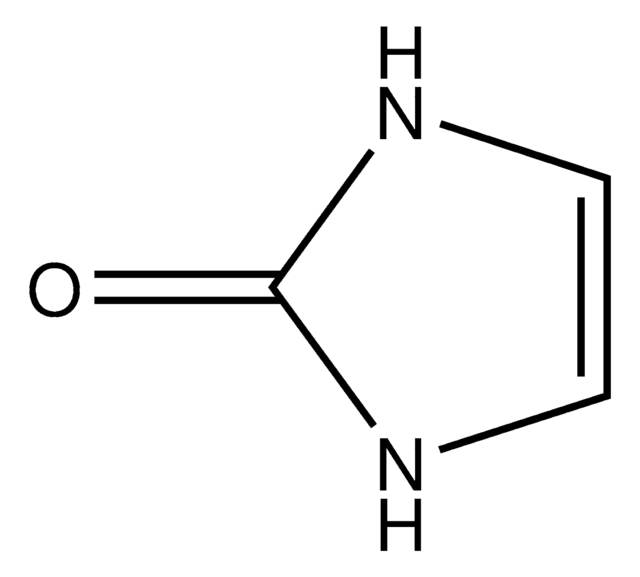
Hydantoin
98%
Synonym(s):
2,4-Imidazolidinedione, Glycolylurea
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C3H4N2O2
CAS Number:
Molecular Weight:
100.08
Beilstein:
110598
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
powder
mp
218-220 °C (lit.)
SMILES string
O=C1CNC(=O)N1
InChI
1S/C3H4N2O2/c6-2-1-4-3(7)5-2/h1H2,(H2,4,5,6,7)
InChI key
WJRBRSLFGCUECM-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Reactant for synthesis of:
N-benzyl aplysinopsin analogs as anticancer agents
D-glutamic acid based inhibitors
Antidiabetic chromonyl-2,4-thiazolidinediones
GSK-3β inhibitors with brain permeability
Thiazolidinedione derivatives as 15-PGDH inhibitors
Radio-sensitizing agents
N-benzyl aplysinopsin analogs as anticancer agents
D-glutamic acid based inhibitors
Antidiabetic chromonyl-2,4-thiazolidinediones
GSK-3β inhibitors with brain permeability
Thiazolidinedione derivatives as 15-PGDH inhibitors
Radio-sensitizing agents
The product has been used as a substrate (at 40 °C and pH 9.0) to determine the D-hydantoinase activity in adzuki bean extract.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Narsimha Reddy Penthala et al.
Bioorganic & medicinal chemistry letters, 21(5), 1411-1413 (2011-02-08)
A series of novel substituted (Z)-5-((1-benzyl-1H-indol-3-yl)methylene)imidazolidin-2,4-diones (3a-f) and (Z)-5-((1-benzyl-1H-indol-3-yl)methylene)-2-iminothiazolidin-4-ones (3g-o) have been synthesized utilizing microwave irradiation. These analogs were evaluated for in vitro cytotoxicity against a panel of 60 human tumor cell lines. Compound 3i exhibits potent growth inhibition against
Ying Wu et al.
Bioorganic & medicinal chemistry, 18(4), 1428-1433 (2010-02-04)
Prostaglandins have a short life in vivo because they are metabolized rapidly by oxidation to 15-ketoprostaglandins catalyzed by a cytosolic enzyme known as NAD(+)-dependent 15-hydroxyprostaglandin dehydrogenase (15-PGDH). Previously, CT-8, a thiazolidinedione analogue, was found to be a potent inhibitor of
Y Thirupathi Reddy et al.
Bioorganic & medicinal chemistry letters, 20(2), 600-602 (2009-12-17)
A series of (Z)-5-((N-benzyl-1H-indol-3-yl)methylene)imidazolidine-2,4-dione (9a-9m) and 5-((N-benzyl-1H-indol-3-yl)methylene)pyrimidine-2,4,6(1H,3H,5H)-trione (10a-10i) derivatives that incorporate a variety of aromatic substituents in both the indole and N-benzyl moieties have been synthesized. These analogs were evaluated for their radiosensitization activity against the HT-29 cell line. Three
June Izquierdo et al.
Chemistry (Weinheim an der Bergstrasse, Germany), 25(53), 12431-12438 (2019-07-19)
A bifunctional amine/squaramide catalyst promoted direct aldol addition of an hydantoin surrogate to pyridine 2-carbaldehyde N-oxides to afford adducts bearing two vicinal tertiary/quaternary carbons in high diastereo- and enantioselectivity (d.r. up to >20:1; ee up to 98 %) is reported. Acid
Effect of treatment with compressed CO2 and propane on D-hydantoinase activity
Andrade JM, et al.
Journal of Supercritical Fluids, 46(2), 342-350 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service