All Photos(3)
About This Item
Empirical Formula (Hill Notation):
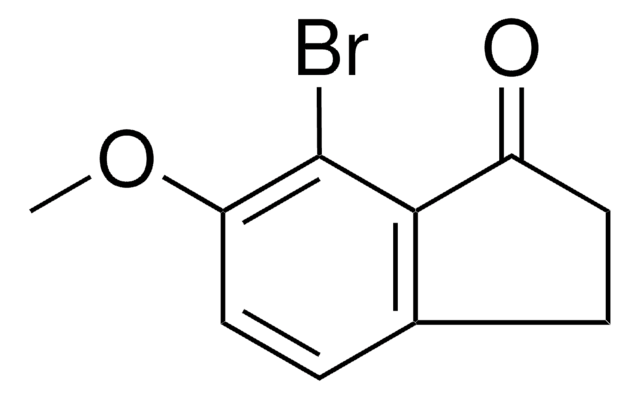
C9H7BrO
CAS Number:
Molecular Weight:
211.06
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
mp
126-129 °C (lit.)
SMILES string
Brc1ccc2C(=O)CCc2c1
InChI
1S/C9H7BrO/c10-7-2-3-8-6(5-7)1-4-9(8)11/h2-3,5H,1,4H2
InChI key
KSONICAHAPRCMV-UHFFFAOYSA-N
Related Categories
General description
5-Bromo-1-indanone is a 1-indanone derivative. Its physical properties like density, freezing point and refractive index have been determined. It participates in the synthesis of the imidazolyl and triazolyl substituted biphenyl compounds.
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 4 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Dawei Ma et al.
The Journal of organic chemistry, 64(1), 120-125 (2001-10-25)
Heating a mixture of alpha-aryl ketone with (R)-phenylglycinol produces a mixture of imine and 1,3-dioxazolidine. Treatment of this mixture with trimethylsilyl cyanide followed by transformation of nitrile to ester gives Strecker-type reaction products. The diastereoselectivity of the generated alpha-amino esters
Andrew K Takle et al.
Bioorganic & medicinal chemistry letters, 16(2), 378-381 (2005-11-02)
A novel triarylimidazole derivative, SB-590885 (33), bearing a 2,3-dihydro-1H-inden-1-one oxime substituent has been identified as a potent and extremely selective inhibitor of B-Raf kinase.
Agustin Casimiro-Garcia et al.
Journal of medicinal chemistry, 54(12), 4219-4233 (2011-05-12)
Mining of an in-house collection of angiotensin II type 1 receptor antagonists to identify compounds with activity at the peroxisome proliferator-activated receptor-γ (PPARγ) revealed a new series of imidazo[4,5-b]pyridines 2 possessing activity at these two receptors. Early availability of the
Turn-On Fluorogenic Probes for the Selective and Quantitative Detection of the Cyanide Anion from Natural Sources.
Gomez T, et al.
Chemistry (Weinheim An Der Bergstrasse, Germany), 8(6), 1271-1278 (2013)
Brian E Fink et al.
Bioorganic & medicinal chemistry letters, 16(6), 1532-1536 (2006-01-03)
A novel series of 17beta-hydroxysteroid dehydrogenase type 3 (17beta-HSD3) inhibitors has been identified. These inhibitors, based on a dibenzazocine core, exhibited picomolar to low nanomolar inhibition of 17beta-HSD3 in cell-free enzymatic as well as in cell-based transcriptional reporter assays.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service