All Photos(1)
About This Item
Empirical Formula (Hill Notation):
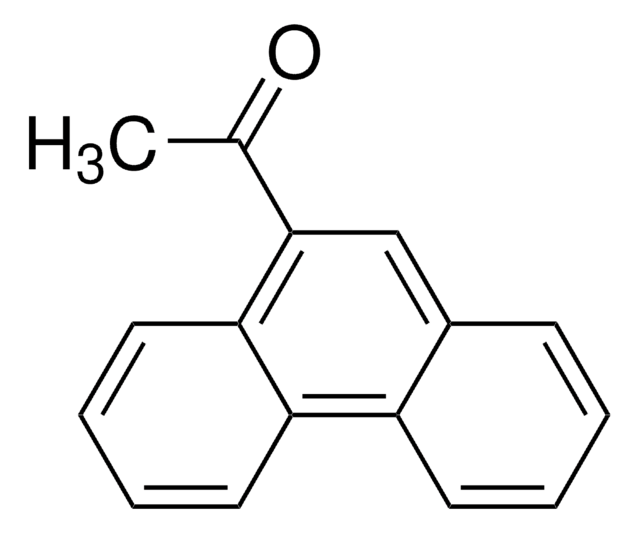
C18H12O
CAS Number:
Molecular Weight:
244.29
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
97%
form
solid
mp
86-89 °C (lit.)
SMILES string
CC(=O)c1ccc2ccc3cccc4ccc1c2c34
InChI
1S/C18H12O/c1-11(19)15-9-7-14-6-5-12-3-2-4-13-8-10-16(15)18(14)17(12)13/h2-10H,1H3
InChI key
KCIJNJVCFPSUBQ-UHFFFAOYSA-N
General description
1-Acetylpyrene is a pyrene derivative. Its synthesis has been reported. Its phytophysical properties have been studied using absolute fluorescence quantum yield measurement and time-dependent density functional theory (TD-DFT) calculations. Its ability to interact with human cytochromes P450 2A13, 2A6, and 1B1 and enzyme inhibition has been reported. Its function as an environment-sensitive fluorophore has been investigated.
Application
1-Acetylpyrene is suitable for use in a comparative study on the photoinitiating efficiency of pyrene, 1-acetylpyrene and 1-(bromoacetyl)pyrene for copolymerization of styrene with acrylonitrile. It may be used in the following studies:
- As a starting material in the synthesis of ethynlypyrene. 1-(1-chlorovinyl)pyrene was also isolated during this reaction.
- As a starting material in the synthesis of substituted pyrene derivatives incorporated heterocyclic and sugar moieties.
- Synthesis of (E)-pyrene oxime ester conjugates of carboxylic acids.
- Synthesis of tertiary alcohols based on 1-acetylpyrene.
- Synthesis of (E)-N-[1-(pyren-1-yl)ethylidene]chrysene-2-amine.
- Synthesis of 3,3-di(methylsulfanyl)-1-(1-pyrenyl)-2-propen-1-one.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis and fluorescent properties of 5-(1-pyrenylethynyl)-2'-deoxyuridine-containing oligodeoxynucleotides.
Malakhov AD, et al.
Russian J. Bioorg. Chem., 26(1), 34-44 (2000)
1-(Bromoacetyl) pyrene, a novel photoinitiator for the copolymerization of styrene and acrylonitrile.
Mishra A and Daswal S.
Coll. Polymer Sci., 285(4), 397-404 (2007)
Fundamental photoluminescence properties of pyrene carbonyl compounds through absolute fluorescence quantum yield measurement and density functional theory.
Niko Y, et al.
Tetrahedron, 68(31), 6177-6185 (2012)
Synthesis and study of film-forming properties and light sensitivity of 4-acyloxy-3-methoxy (ethoxy) phenylmethylidene-(chrysen-2-yl) amines.
Dikusar EA, et al.
Russ. J. Gen. Chem., 77(2), 278-281 (2007)
One-pot synthesis of 1-acetylpyrene over supported phosphotungstic heteropoly acid catalysts.
He MQ, et al.
Reaction Kinetics, Mechanisms and Catalysis, 108(2), 531-544 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service