166952
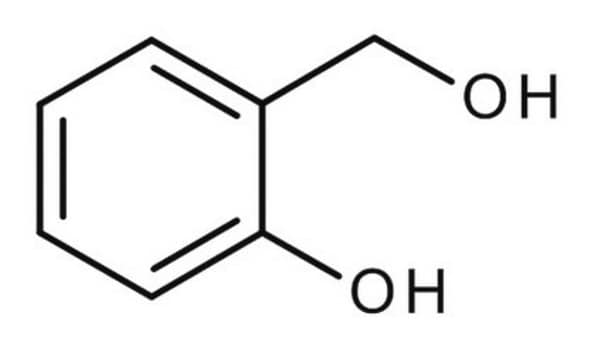
2-Hydroxybenzyl alcohol
99%
Synonym(s):
Salicyl alcohol, Saligenin
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
HOC6H4CH2OH
CAS Number:
Molecular Weight:
124.14
Beilstein:
1907195
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Assay
99%
form
solid
mp
83-85 °C (lit.)
solubility
ethanol: soluble 5%, clear to very slightly hazy, colorless to light yellow
functional group
hydroxyl
SMILES string
OCc1ccccc1O
InChI
1S/C7H8O2/c8-5-6-3-1-2-4-7(6)9/h1-4,8-9H,5H2
InChI key
CQRYARSYNCAZFO-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
General description
2-Hydroxybenzyl alcohol participates in the selective ring opening reaction of 4H-1,3,2-benzodioxasilines.
2-Hydroxybenzyl alcohol can be used as a coupling reagent to synthesize O-heterocycles.
2-Hydroxybenzyl alcohol can be used as a coupling reagent to synthesize O-heterocycles.
Application
2-Hydroxybenzyl alcohol was used in gastrodin production via biotransformation by cultured cells of Aspergillus foetidus and Penicillium cyclopium.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Selective ring opening of 4H-1, 3, 2-benzodioxasiline twin monomers.
Kempe P, et al.
New. J. Chem., 35(12), 2735-2739 (2011)
Catalytic acceptorless dehydrogenation of amino alcohols and 2-hydroxybenzyl alcohols for annulation reaction under neutral conditions
Pandey AM, et al.
The Journal of Organic Chemistry, 86, 8805-8828 (2021)
Linlin Fan et al.
Applied biochemistry and biotechnology, 170(1), 138-148 (2013-03-14)
The objective of this work was to take advantage of the resting cells of suitable fungus as an in vitro model to prepare gastrodin from p-2-hydroxybenzyl alcohol (HBA), which mainly exists in the metabolites of the plant Gastrodia elata Blume.
Fernando A Genta et al.
Journal of insect physiology, 52(6), 593-601 (2006-04-08)
Tenebrio molitor larvae were successfully reared free of cultivatable gut lumen bacteria, yeasts and fungi using two approaches; aseptic rearing from surface sterilized eggs and by feeding larvae with antibiotic-containing food. Insects were reared on a rich-nutrient complete diet or
Nicolas Gisch et al.
Journal of medicinal chemistry, 51(21), 6752-6760 (2008-10-07)
Recently we reported on conceptually new enzymatically activated cycloSal-pronucleotides. Now, we developed this concept further with new compounds of this type. The basic idea is fast intracellular cleavage of a functionalized group at the cycloSal residue that results in a
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service