B5437
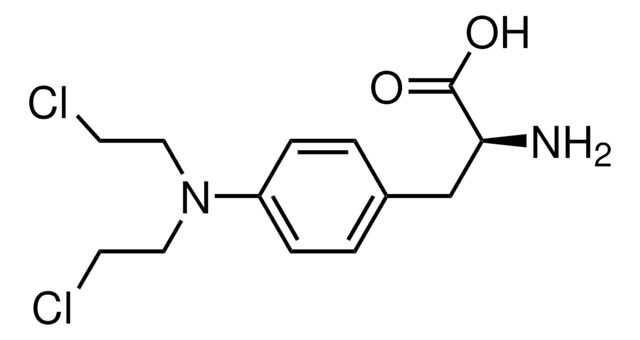
Bendamustine hydrochloride hydrate
≥98% (HPLC)
Synonym(s):
1H-Benzimidazole-2-butanoic acid, 5-[bis(2-chloroethyl)amino]-1-methyl monohydrochloride, Treanda
About This Item
Recommended Products
Quality Level
Assay
≥98% (HPLC)
form
powder
storage condition
desiccated
color
off-white
solubility
H2O: >30 mg/mL
originator
Teva
storage temp.
room temp
SMILES string
O.Cl.Cn1c(CCCC(O)=O)nc2cc(ccc12)N(CCCl)CCCl
InChI
1S/C16H21Cl2N3O2.ClH.H2O/c1-20-14-6-5-12(21(9-7-17)10-8-18)11-13(14)19-15(20)3-2-4-16(22)23;;/h5-6,11H,2-4,7-10H2,1H3,(H,22,23);1H;1H2
InChI key
TWBJYCLUHINEDN-UHFFFAOYSA-N
General description
Application
- a chemotherapy agent for chronic lymphocytic leukemia (CLL) samples to monitor spliced and unspliced gene expression
- an inhibitor to E3 ubiquitin-protein ligase RNF3 (HOIP) in matrix-assisted laser desorption ionization time-of-flight mass spectrometry (MALDI-TOF) assay
- a cytotoxic chemotherapeutic drug in high-throughput screening to test interaction with BAY87-2243
Biochem/physiol Actions
Features and Benefits
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Carc. 2 - Muta. 2 - Repr. 2
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Articles
Apoptosis regulation involves multiple pathways and molecules for cellular homeostasis.
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
Cell cycle phases (G1, S, G2, M) regulate cell growth, DNA replication, and division in proliferating cells.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service