60372
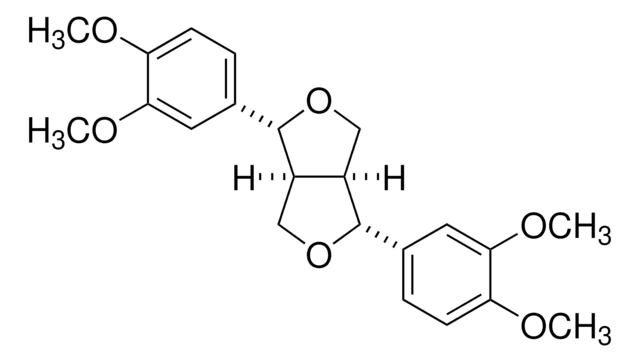
Secoisolariciresinol
≥95.0% (HPLC)
Synonym(s):
(2R,3R)-2,3-Bis(4-hydroxy-3-methoxybenzyl)-1,4-butanediol
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C20H26O6
Molecular Weight:
362.42
Beilstein:
6611290
EC Number:
MDL number:
UNSPSC Code:
41116105
PubChem Substance ID:
NACRES:
NA.21
Recommended Products
Quality Level
Assay
≥95.0% (HPLC)
form
solid
SMILES string
OC[C@@H]([C@H](CO)CC1=CC=C(O)C(OC)=C1)CC2=CC(OC)=C(O)C=C2
InChI
1S/C20H26O6/c1-25-19-9-13(3-5-17(19)23)7-15(11-21)16(12-22)8-14-4-6-18(24)20(10-14)26-2/h3-6,9-10,15-16,21-24H,7-8,11-12H2,1-2H3/t15-,16-/m0/s1
InChI key
PUETUDUXMCLALY-HOTGVXAUSA-N
Application
Secoisolariciresinol is a metabolite of secoisolariciresinol diglucoside (SDG), an antioxidant present in flaxseed.
Packaging
Bottomless glass bottle. Contents are inside inserted fused cone.
Physical form
mixture of enantiomers of the (R*,R*)-diastereoisomer
Other Notes
Component of functional food. Flaxseed lignan with antioxidant activity
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Cheng-Zhi Wang et al.
BMC microbiology, 10, 115-115 (2010-04-20)
The effects of enterolignans, e.g., enterodiol (END) and particularly its oxidation product, enterolactone (ENL), on prevention of hormone-dependent diseases, such as osteoporosis, cardiovascular diseases, hyperlipemia, breast cancer, colon cancer, prostate cancer and menopausal syndrome, have attracted much attention. To date
D D Kitts et al.
Molecular and cellular biochemistry, 202(1-2), 91-100 (2000-03-08)
The antioxidant activities of the flaxseed lignan secoisolariciresinol diglycoside (SDG) and its mammalian lignan metabolites, enterodiol (ED) and enterolactone (EL), were evaluated in both lipid and aqueous in vitro model systems. All three lignans significantly (p < or = 0.05)
Shiori Tominaga et al.
Food & function, 3(1), 76-82 (2011-10-28)
Flaxseed lignan, secoisolariciresinol has been reported to possess health benefits. We previously synthesized each stereoisomer of secoisolariciresinol and found that (-)-secoisolariciresinol reduces lipid accumulation and induces adiponectin production in 3T3-L1 adipocytes. Here we show the effects of (-)-secoisolariciresinol on high-fat
K Struijs et al.
Journal of applied microbiology, 107(1), 308-317 (2009-03-24)
It has been investigated whether secoisolariciresinol (SECO) and anhydrosecoisolariciresinol (AHS), an acid degradation product of SECO, could be fermented in a similar way, and to a similar extent, by members of the intestinal microbiota. AHS and SECO were demethylated by
Sandra M Sacco et al.
Journal of medicinal food, 14(10), 1208-1214 (2011-06-15)
Flaxseed, rich in the phytoestrogen lignan secoisolariciresinol diglycoside (SDG), provides protection against bone loss at the lumbar vertebrae primarily when combined with low-dose estrogen therapy in the ovariectomized rat model of postmenopausal osteoporosis. Whether SDG metabolites are accessible to skeletal
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service