8.09692
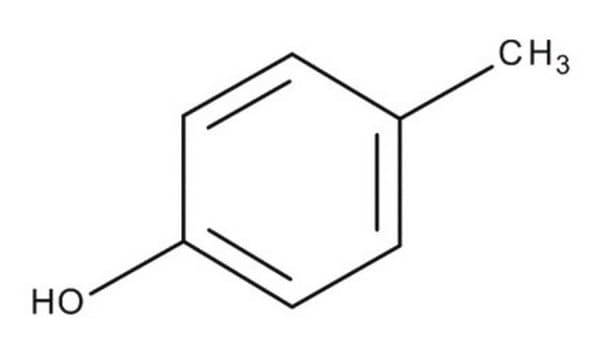
o-Cresol
for synthesis
Synonym(s):
o-Cresol, 2-Hydroxytoluene, 2-Methylphenol
About This Item
Recommended Products
vapor pressure
0.24 hPa ( 20 °C)
Quality Level
Assay
≥99% (GC)
form
solid
autoignition temp.
555 °C
potency
121 mg/kg LD50, oral (Rat)
890 mg/kg LD50, skin (Rabbit)
expl. lim.
1.3 % (v/v)
pH
4.8 (20 °C, 20 g/L in H2O)
bp
191-192 °C/1013 hPa
mp
31 °C
transition temp
flash point 81 °C
solubility
20 g/L
density
1.046 g/cm3 at 20 °C
storage temp.
no temp limit
InChI
1S/C7H8O/c1-6-4-2-3-5-7(6)8/h2-5,8H,1H3
InChI key
QWVGKYWNOKOFNN-UHFFFAOYSA-N
Application
- Carvacrol by alkylation reaction with propylene or isopropyl alcohol in the presence of Zr/mesoporous silica as a catalyst.
- Polyisobutylenes by selective cationic polymerization of isobutylene using AlCl3 as a catalyst.
- Poly(o-cresol) via oxidative coupling polymerization reaction in the presence of substituted pyridine/CuCl catalysts.
Analysis Note
Melting range (lower value): ≥ 29 °C
Melting range (upper value): ≤ 33 °C
Water (K. F.): ≤ 0.10 %
Identity (IR): passes test
Due to its specific melting range the product may be solid, liquid, a solidified melt or a supercooled melt.
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Dermal - Acute Tox. 3 Oral - Aquatic Chronic 3 - Eye Dam. 1 - Skin Corr. 1B
Storage Class Code
6.1A - Combustible acute toxic Cat. 1 and 2 / very toxic hazardous materials
WGK
WGK 2
Flash Point(F)
177.8 °F - closed cup
Flash Point(C)
81.0 °C - closed cup
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service