790528P
Avanti
18:1 DGS-NTA
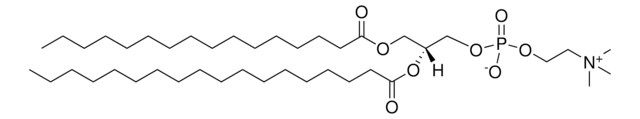
1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (ammonium salt), powder
Synonym(s):
1,2-di-(9Z-octadecenoyl)-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (ammonium salt); DOGS NTA
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Empirical Formula (Hill Notation):
C53H100N5O13
CAS Number:
Molecular Weight:
1015.39
UNSPSC Code:
12352211
NACRES:
NA.25
Recommended Products
Assay
>99% (TLC)
form
powder
packaging
pkg of 1 × 5 mg (790528P-5mg)
manufacturer/tradename
Avanti Research™ - A Croda Brand 790528P
shipped in
dry ice
storage temp.
−20°C
General description
1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (18:1 DGS-NTA) is amphiphile. DGS-NTA is a nitrilotriacetic acid -functionalized amphiphile devoid of nickel ion.
Application
18:1 DGS-NTA 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (ammonium salt) has been used:
- for generation of amphiphile monolayer
- in NTA-liposome preparation for procoagulant binding studies and to study its effect on factor FXII autoactivation
- as a liposome component for lipid bilayer preparation for surface plasmon resonance studies
Biochem/physiol Actions
1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (18:1 DGS-NTA or DOGS-NTA) cannot bind to histidine due to lack of nickel ion. It is capable of self-assembly to form a stable monolayer. DOGS-NTA ammonium salt is useful in bicelle preparation.
Packaging
5 mL Clear Glass Sealed Ampule (790528P-5mg)
Legal Information
Avanti Research is a trademark of Avanti Polar Lipids, LLC
Storage Class Code
11 - Combustible Solids
Certificates of Analysis (COA)
Search for Certificates of Analysis (COA) by entering the products Lot/Batch Number. Lot and Batch Numbers can be found on a product’s label following the words ‘Lot’ or ‘Batch’.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Ronald D Seidel et al.
Journal of the American Chemical Society, 129(15), 4834-4839 (2007-03-28)
The study of bound-state conformations of ligands interacting with proteins is important to the understanding of protein function and the design of drugs that alter function. Traditionally, transferred nuclear Overhauser effects (trNOEs), measured from NMR spectra of ligands in rapid
Louis Gioia et al.
Science immunology, 4(38) (2019-09-01)
The class II region of the major histocompatibility complex (MHC) locus is the main contributor to the genetic susceptibility to type 1 diabetes (T1D). The loss of an aspartic acid at position 57 of diabetogenic HLA-DQβ chains supports this association;
Decorating liquid crystal surfaces with proteins for real-time detection of specific protein-protein binding
Hartono D, et al.
Advances in Functional Materials, 19(22), 3574-3579 (2009)
N J Mutch et al.
Journal of thrombosis and haemostasis : JTH, 10(10), 2108-2115 (2012-08-22)
Upon contact with an appropriate surface, factor XII (FXII) undergoes autoactivation or cleavage by kallikrein. Zn(2+) is known to facilitate binding of FXII and the cofactor, high molecular weight kininogen (HK), to anionic surfaces. To investigate whether transition metal ions
E M Wilson-Kubalek et al.
Proceedings of the National Academy of Sciences of the United States of America, 95(14), 8040-8045 (1998-07-08)
A general approach for crystallization of proteins in a fast and simple manner would be of immense interest to biologists studying protein structure-function relationships. Here, we describe a method that we have developed for promoting the formation of helical arrays
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service


![18:1 DGS-NTA(Co) 1,2-dioleoyl-sn-glycero-3-[(N-(5-amino-1-carboxypentyl)iminodiacetic acid)succinyl] (Cobalt salt), powder](/deepweb/assets/sigmaaldrich/product/structures/203/482/b877a7a9-e8a9-4cec-99cf-b256a26d690c/640/b877a7a9-e8a9-4cec-99cf-b256a26d690c.png)