All Photos(3)
About This Item
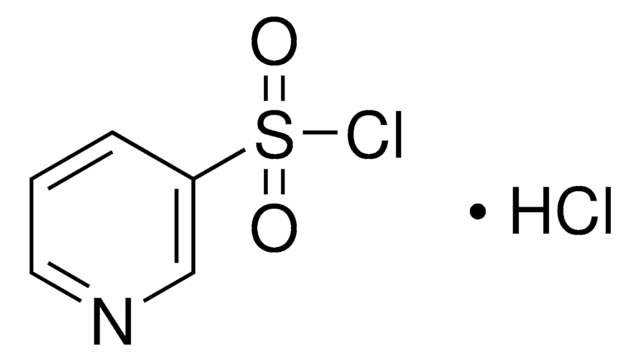
Empirical Formula (Hill Notation):
C9H6ClNO2S
CAS Number:
Molecular Weight:
227.67
Beilstein:
156347
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
mp
126-129 °C (lit.)
SMILES string
ClS(=O)(=O)c1cccc2cccnc12
InChI
1S/C9H6ClNO2S/c10-14(12,13)8-5-1-3-7-4-2-6-11-9(7)8/h1-6H
InChI key
JUYUYCIJACTHMK-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
J Borras et al.
Bioorganic & medicinal chemistry, 7(11), 2397-2406 (2000-01-13)
Reaction of 20 aromatic/heterocyclic sulfonamides containing a free amino, imino, hydrazino or hydroxyl group, with 8-quinoline-sulfonyl chloride afforded a series of water-soluble (as hydrochloride or triflate salts) compounds. The new derivatives were assayed as inhibitors of the zinc enzyme carbonic
Vijay K Agrawal et al.
European journal of medicinal chemistry, 39(7), 593-600 (2004-07-09)
Quantitative structure-activity-relationship (QSAR) study on aromatic/heterocyclic sulfonamides containing 8-quinoline-sulfonyl carbonic anhydrase (CA) inhibitors has been carried out topologically using first-order valence connectivity index ((1)chi(v)). Excellent results are obtained against all the three isozymes; CA I, II and IV of the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service