O3750
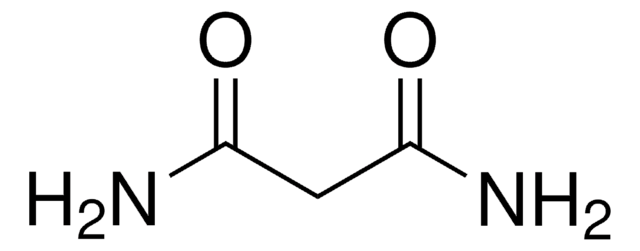
Oxamic acid
≥98%
Synonym(s):
Aminooxoacetic acid, Oxalic acid monoamide
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
NH2COCO2H
CAS Number:
Molecular Weight:
89.05
Beilstein:
1743294
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
≥98%
form
powder
mp
207-210 °C (dec.) (lit.)
SMILES string
NC(=O)C(O)=O
InChI
1S/C2H3NO3/c3-1(4)2(5)6/h(H2,3,4)(H,5,6)
InChI key
SOWBFZRMHSNYGE-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
Oxamic acid (OA) can be used as a reactant to prepare 6-phenanthridinecarboxamide by direct C-H carbamoylation reaction using ammonium persulfate in DMSO. It can also be used as an organic ligand to prepare functionalized metal oxide nanoparticles for various biological applications. OA along with p-aminobenzoic acid is used to functionalize Au nanoparticles for the development of a sensor to detect Fe3+ ions by the calorimetric method.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Metal-, Photocatalyst-, and Light-Free Direct C-H Acylation and Carbamoylation of Heterocycles
Westwood MT, et al.
Organic Letters, 21(17), 7119-7123 (2019)
Xuguang Yang et al.
Cancer immunology research, 8(11), 1440-1451 (2020-09-13)
The mechanisms responsible for radioresistance in pancreatic cancer have yet to be elucidated, and the suppressive tumor immune microenvironment must be considered. We investigated whether the radiotherapy-augmented Warburg effect helped myeloid cells acquire an immunosuppressive phenotype, resulting in limited treatment
Carboxylic acid-stabilised iron oxide nanoparticles for use in magnetic hyperthermia
Thomas, Luanne A, et al.
Journal of Materials Chemistry, 19(36), 6529-6535 (2009)
Camille Vandenberghe et al.
Frontiers in nutrition, 7, 3-3 (2020-02-23)
Ketones provide an alternative brain fuel and may be neuroprotective in older people. Little is known of how to optimize the ketogenic effect of C8:0-C10:0 medium chain triglyceride supplement (kMCT). Metabolic switching (MS) from glucose to ketones as a fuel
Xiao-Wen Li et al.
European journal of medicinal chemistry, 46(9), 3851-3857 (2011-06-15)
A novel dissymmetrical N,N'-bis(substituted)oxamide ligand, N-(2-aminopropyl)-N'-(2-oxido- phenyl)oxamide (H(3)apopoxd) (L), and its three bicopper(II) complexes, [Cu(2)(apopoxd)(bpy)]- (ClO(4))·H(2)O (1), [Cu(2)(apopoxd)(dabt)](ClO(4))·2H(2)O (2), and [Cu(2)(apopoxd)(phen)(2)](ClO(4)) (3) (bpy = 2,2'-bipyridine; dabt = 2,2'-diamino-4,4'-bithiazole; phen = 1,10-phenanthroline) have been synthesized and characterized. The crystal structures of
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service