C86405
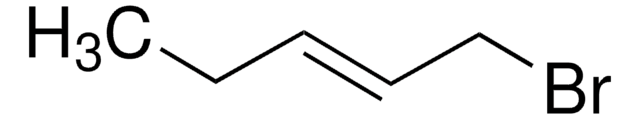
Crotyl bromide
technical grade, 85% (mixture of cis & trans)
Synonym(s):
trans-1-Bromo-2-butene
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
CH3CH=CHCH2Br
CAS Number:
Molecular Weight:
135.00
Beilstein:
1361394
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
grade
technical grade
Quality Level
Assay
85% (mixture of cis & trans)
form
liquid
refractive index
n20/D 1.480 (lit.)
bp
97-99 °C (lit.)
density
1.312 g/mL at 25 °C (lit.)
storage temp.
2-8°C
SMILES string
[H]\C(C)=C(\[H])CBr
InChI
1S/C4H7Br/c1-2-3-4-5/h2-3H,4H2,1H3/b3-2+
InChI key
AVMHMVJVHYGDOO-NSCUHMNNSA-N
Looking for similar products? Visit Product Comparison Guide
Related Categories
Application
Crotyl bromide is a general organic reagent that can be used in the total synthesis of biologically important natural products and their derivatives such as (+)-artemisinin, plakortone E and (−)-horsfiline.
It can also be used to prepare analogs of deoxycyclitols, pentopyranoses, 6-deoxyhexoses, hexoses and pseudo-indoxyl derivatives.
It can also be used to prepare analogs of deoxycyclitols, pentopyranoses, 6-deoxyhexoses, hexoses and pseudo-indoxyl derivatives.
Other Notes
remainder 3-bromo-1-butene
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Flam. Liq. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
3 - Flammable liquids
WGK
WGK 3
Flash Point(F)
51.8 °F - closed cup
Flash Point(C)
11 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
A potentially divergent and rapid route to analogues of deoxycyclitols, pentopyranoses, 6-deoxyhexoses, and hexoses.
Audouard C.
Organic Letters, 6(23), 4269-4272 (2004)
Efficient Enantioselective Total Synthesis of (−)?Horsfiline.
Hong S.
Chemistry?A European Journal , 19(29), 9599-9605 (2013)
Total synthesis and absolute stereochemistry of plakortone E.
Akiyama M.
Tetrahedron Letters, 47(14), 2287-2290 (2006)
A concise synthesis of (+)-artemisinin.
Zhu C and Cook SP.
Journal of the American Chemical Society, 134(33), 13577-13579 (2012)
Synthesis of pseudo-indoxyl derivatives via sequential Cu-catalyzed SN Ar and Smalley cyclization.
Goriya Y and Ramana CV.
Chemical Communications (Cambridge, England), 49(57), 6376-6378 (2013)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service