C65408
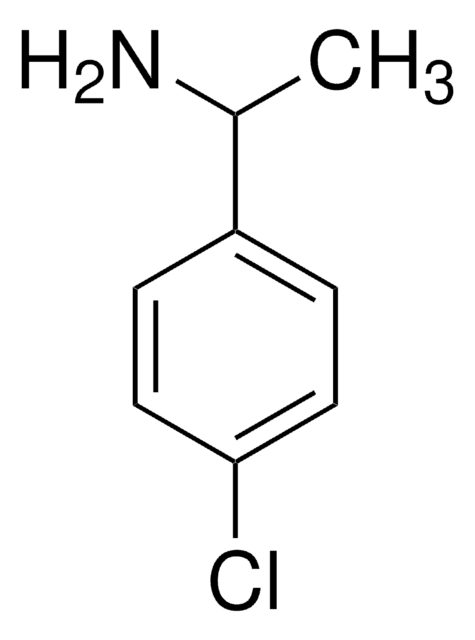
2-(4-Chlorophenyl)ethylamine
98%
Synonym(s):
4-Chlorophenethylamine
Sign Into View Organizational & Contract Pricing
All Photos(1)
About This Item
Linear Formula:
ClC6H4CH2CH2NH2
CAS Number:
Molecular Weight:
155.62
Beilstein:
508247
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
liquid
refractive index
n20/D 1.548 (lit.)
bp
60-65 °C/0.1 mmHg (lit.)
density
1.112 g/mL at 25 °C (lit.)
SMILES string
NCCc1ccc(Cl)cc1
InChI
1S/C8H10ClN/c9-8-3-1-7(2-4-8)5-6-10/h1-4H,5-6,10H2
InChI key
SRXFXCKTIGELTI-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
10 - Combustible liquids
WGK
WGK 3
Flash Point(F)
222.8 °F - closed cup
Flash Point(C)
106 °C - closed cup
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
E Chung Hwang et al.
The Journal of pharmacology and experimental therapeutics, 213(2), 254-260 (1980-05-01)
A series of substituted phenylethylamines were examined for their relative potencies to 1) release and inhibit accumulation of labeled serotonin in brain synaptosomes, 2) compete with serotonin receptor binding and 3) induce the "serotonin syndrome" in mice. In general, the
T N Mellin et al.
The American journal of tropical medicine and hygiene, 32(1), 83-93 (1983-01-01)
Neuropharmacological studies of Schistosoma mansoni were conducted in vitro using visual observations of motor activity and measurements of worm length and extracellular electrical activity. The instrumentation and methodology described quantitatively measure extracellular electrical potentials associated with motor activity, and provide
Giulia Guiducci et al.
Nucleic acids research, 47(8), 4240-4254 (2019-02-28)
Enzymes of intermediary metabolism are often reported to have moonlighting functions as RNA-binding proteins and have regulatory roles beyond their primary activities. Human serine hydroxymethyltransferase (SHMT) is essential for the one-carbon metabolism, which sustains growth and proliferation in normal and
G B Baker et al.
Progress in neuro-psychopharmacology & biological psychiatry, 6(4-6), 343-346 (1982-01-01)
1. The accumulation of p-chlorophenylethylamine (pCPE) in rat brain after administration of pargyline plus p-chlorophenylalanine (pCPA) is demonstrated. 2. Measurements of pCPE in brain were performed at 0.25 h, 1 h and 4 h after administration of pCPA to pargyline-pretreated
Khalid Touiki et al.
Psychopharmacology, 182(4), 562-569 (2005-09-01)
Harmane and norharmane (two beta-carbolines) are tobacco components or products. The effects of harmane and norharmane on serotonergic raphe neurons remain unknown. Harmane and norharmane are inhibitors of the monoamine oxidases A (MAO-A) and B (MAO-B), respectively. To study the
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service