All Photos(3)
About This Item
Empirical Formula (Hill Notation):
C7H7N3
CAS Number:
Molecular Weight:
133.15
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
175-178 °C (lit.)
SMILES string
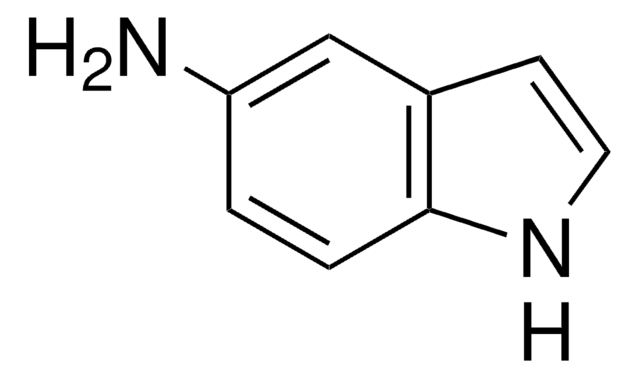
Nc1ccc2[nH]ncc2c1
InChI
1S/C7H7N3/c8-6-1-2-7-5(3-6)4-9-10-7/h1-4H,8H2,(H,9,10)
InChI key
XBTOSRUBOXQWBO-UHFFFAOYSA-N
Related Categories
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Acute Tox. 3 Oral - Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
6.1C - Combustible acute toxic Cat.3 / toxic compounds or compounds which causing chronic effects
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Chai Hoon Soh et al.
Journal of combinatorial chemistry, 8(4), 464-468 (2006-07-11)
A microwave-assisted parallel synthesis of 2,4-disubstituted 5-aminoimidazoles has been developed. Significant rate enhancement was observed for all steps in the three-step protocol. The overall reaction time was shortened to 25 min, as compared to 53 h for the conventional procedures.
Feng Shi et al.
Molecular diversity, 15(2), 497-505 (2010-09-04)
Six new series of pyrazolo[4,3-f]quinoline derivatives with potential bioactivities were synthesized by the three-component reactions of aromatic aldehydes, 5-aminoindazole, and various cyclic 1,3-dicarbonyl compounds under microwave irradiation. This protocol has the valuable features of structural diversity of products, broader substrate
Ratika Krishnamurty et al.
Bioorganic & medicinal chemistry letters, 21(1), 550-554 (2010-11-17)
Affinity reagents that target protein kinases are powerful tools for signal transduction research. Here, we describe a general set of kinase ligands based on a 5-aminoindazole scaffold. This scaffold can readily be derivatized with diverse binding elements and immobilized analogs
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service