859796
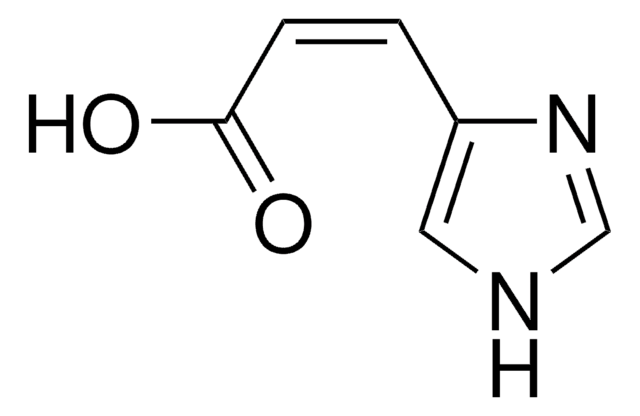
4-Imidazoleacrylic acid
99%
Synonym(s):
3-(4-Imidazolyl)acrylic acid, Urocanic acid
Sign Into View Organizational & Contract Pricing
All Photos(5)
About This Item
Empirical Formula (Hill Notation):
C6H6N2O2
CAS Number:
Molecular Weight:
138.12
Beilstein:
81405
EC Number:
MDL number:
UNSPSC Code:
12352005
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
biological source
synthetic
Assay
99%
form
powder
mp
226-228 °C (lit.)
functional group
carboxylic acid
SMILES string
OC(=O)\C=C\c1c[nH]cn1
InChI
1S/C6H6N2O2/c9-6(10)2-1-5-3-7-4-8-5/h1-4H,(H,7,8)(H,9,10)/b2-1+
InChI key
LOIYMIARKYCTBW-OWOJBTEDSA-N
Looking for similar products? Visit Product Comparison Guide
General description
4-Imidazoleacrylic acid also known as urocanic acid is a natural metabolite derived from histidine. It is majorly used as a UV chromophore with a strong absorption spectrum in the UV-B region in the range of 300-280 nm.
Application
4-Imidazoleacrylic acid can be used as a precursor for the synthesis of (±)-homohistidine, urocanic acid-modified chitosan, and N1-aryl(heteroaryl)alkyl-N2-[3-(1H-imidazol-4-yl)propyl]guanidines,.
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
Synthesis of (?)-homohistidine
Pirrung MC and Pei T
The Journal of Organic Chemistry, 65(7), 2229-2230 (2000)
Anne J Keurentjes et al.
Frontiers in public health, 9, 602933-602933 (2021-03-23)
Introduction: Non-melanoma skin cancer (NMSC) incidence is increasing, and occupational solar exposure contributes greatly to the overall lifetime ultraviolet radiation (UVR) dose. This is reflected in an excess risk of NMSC showing up to three-fold increase in outdoor workers. Risk
Sabita Rana et al.
The American journal of pathology, 178(6), 2783-2791 (2011-06-07)
Exposure to UVB radiation before antigen delivery at an unirradiated site inhibits functional immunological responses. Mice treated dorsally with suberythemal low-dose UVB and immunized with ova in abdominal skin generated ova-specific CD8 T cells with a significantly decreased activation, expansion
Peter D Godfrey et al.
The Journal of chemical physics, 137(6), 064306-064306 (2012-08-18)
The microwave spectra of the two conformers each, of the 1H and 3H tautomers of 4-vinylimidazole, have been measured in the 48-72 GHz spectral region. The 4-vinylimidazole was generated in situ by the facile decarboxylation of urocanic acid at its
Acylguanidines as bioisosteres of guanidines: N G-acylated imidazolylpropylguanidines, a new class of histamine H2 receptor agonists
Ghorai P, et al.
Journal of Medicinal Chemistry, 51(22), 7193-7204 (2008)
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service