858781
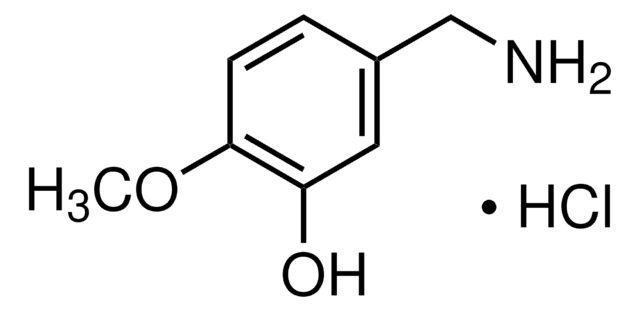
3,4-Dihydroxybenzylamine hydrobromide
98%
Synonym(s):
4-(Aminomethyl)catechol hydrobromide, DHBA hydrobromide
Sign Into View Organizational & Contract Pricing
All Photos(3)
About This Item
Linear Formula:
(HO)2C6H3CH2NH2 · HBr
CAS Number:
Molecular Weight:
220.06
Beilstein:
4002646
EC Number:
MDL number:
UNSPSC Code:
12352100
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
98%
form
crystals
mp
184-186 °C (lit.)
functional group
amine
SMILES string
Br.NCc1ccc(O)c(O)c1
InChI
1S/C7H9NO2.BrH/c8-4-5-1-2-6(9)7(10)3-5;/h1-3,9-10H,4,8H2;1H
InChI key
BVFZTXFCZAXSHN-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
<ul>
<li><strong>Oxidative Polymerization of 3,4-Dihydroxybenzylamine:</strong> 3,4-Dihydroxybenzylamine is used in the synthesis of poly[3,4-dihydroxybenzylamine] (PDHBA) by oxidative polymerization, exploring its application as a lower homolog of dopamine for potential use in synthetic pathways and materials science (Petran et al., 2023).</li>
<li><strong>Detection of Urinary Free Metanephrines:</strong> Utilizing 3,4-Dihydroxybenzylamine hydrobromide as an internal standard, this research enhances the detection accuracy of urinary free metanephrines for diagnosing pheochromocytomas and paragangliomas, showcasing its importance in clinical diagnostic applications (Wang et al., 2020).</li>
<li><strong>Development of HPLC-ECD Method:</strong> A study developed an HPLC-ECD method using 3,4-Dihydroxybenzylamine as an internal standard for the analysis of vitamin C in plasma, demonstrating the chemical’s utility in enhancing analytical methodologies in biochemical research (Clark and Frank, 2016).</li>
<li><strong>Fluorescence Analysis of Catecholamines:</strong> 3,4-Dihydroxybenzylamine is used as an internal standard to determine catecholamines and related compounds in rat brain tissue, underlining its application in neurochemical analysis and research (Fonseca et al., 2017).</li>
</ul>
<li><strong>Oxidative Polymerization of 3,4-Dihydroxybenzylamine:</strong> 3,4-Dihydroxybenzylamine is used in the synthesis of poly[3,4-dihydroxybenzylamine] (PDHBA) by oxidative polymerization, exploring its application as a lower homolog of dopamine for potential use in synthetic pathways and materials science (Petran et al., 2023).</li>
<li><strong>Detection of Urinary Free Metanephrines:</strong> Utilizing 3,4-Dihydroxybenzylamine hydrobromide as an internal standard, this research enhances the detection accuracy of urinary free metanephrines for diagnosing pheochromocytomas and paragangliomas, showcasing its importance in clinical diagnostic applications (Wang et al., 2020).</li>
<li><strong>Development of HPLC-ECD Method:</strong> A study developed an HPLC-ECD method using 3,4-Dihydroxybenzylamine as an internal standard for the analysis of vitamin C in plasma, demonstrating the chemical’s utility in enhancing analytical methodologies in biochemical research (Clark and Frank, 2016).</li>
<li><strong>Fluorescence Analysis of Catecholamines:</strong> 3,4-Dihydroxybenzylamine is used as an internal standard to determine catecholamines and related compounds in rat brain tissue, underlining its application in neurochemical analysis and research (Fonseca et al., 2017).</li>
</ul>
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2 - Skin Irrit. 2 - STOT SE 3
Target Organs
Respiratory system
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Personal Protective Equipment
dust mask type N95 (US), Eyeshields, Gloves
Choose from one of the most recent versions:
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Customers Also Viewed
F Boomsma et al.
Journal of chromatography, 621(1), 82-88 (1993-11-17)
We report a rapid breakdown of dopamine and especially of 3,4-dihydroxybenzylamine, the frequently-used internal standard in catecholamine determinations, in plasma of many but not all animal species. Species investigated were cow, sheep, goat, pig, horse, rabbit, dog, guinea pig, mouse
Sophie Bourcier et al.
European journal of mass spectrometry (Chichester, England), 9(4), 351-360 (2003-08-27)
Our research into neurotransmitters in a biological fluid presented an opportunity to investigate the fragmentations under low collision energy characterising benzyl-amines protonated under electrospray ionisation (ESI) conditions in a triple quadrupole mass spectrometer. In this work we present the breakdown
F Boomsma et al.
Journal of cardiovascular pharmacology, 22(2), 198-202 (1993-08-01)
We noted rapid breakdown at 4 degrees and 20 degrees C of dopamine (DA) (but not of (nor)epinephrine and epinine) in pig plasma, but not in human plasma. The enzyme responsible appears to be a semicarbazide-sensitive amine oxidase (SSAO) because
E C Chan et al.
Rapid communications in mass spectrometry : RCM, 14(21), 1959-1964 (2000-11-21)
An assay of norepinephrine (NE), epinephrine (E), dopamine (DA), normetanephrine (NE) and metanephrine (MN) based on high-performance liquid chromatography (HPLC) in combination with atmospheric pressure chemical ionization mass spectrometry (APcI-MS) is described. The catecholamines and metanephrines were extracted from urine
J A Prezioso et al.
Pigment cell research, 3(2), 49-54 (1990-03-01)
The rationale for melanoma specific dihydroxybenzene containing antitumor agents is based in part upon the ability of the enzyme tyrosinase to oxidize these pro drugs to toxic intermediates. In situ tyrosinase activity was demonstrated to be affected by both cell
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service