84739
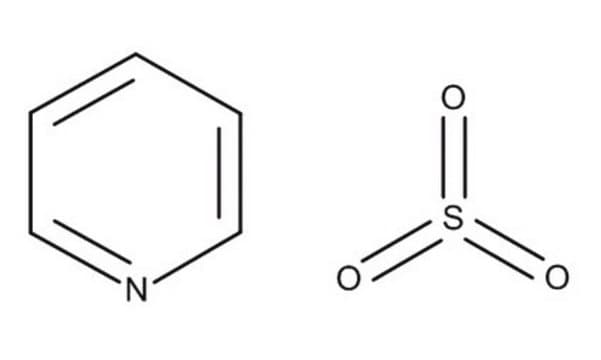
Sulfur trioxide triethylamine complex
technical, ≥95% sulfur basis
Synonym(s):
NSC 68185, NSC 8168, Triethylamine sulfur trioxide complex
About This Item
Recommended Products
grade
technical
Quality Level
Assay
≥95% sulfur basis
form
powder
reaction suitability
reagent type: oxidant
mp
~85 °C
functional group
amine
storage temp.
2-8°C
SMILES string
O=S(=O)=O.CCN(CC)CC
InChI
1S/C6H15N.O3S/c1-4-7(5-2)6-3;1-4(2)3/h4-6H2,1-3H3;
InChI key
YYHPEVZFVMVUNJ-UHFFFAOYSA-N
Looking for similar products? Visit Product Comparison Guide
Application
- Anticoagulant and antiplatelet persulfated glycosides or flavonoids containing an oligopolysulfated moiety
- Narciclasine derivatives for use as anticancer agents
- Sulfate-conjugated methylprednisolone for use as a colon-specific prodrug
- Tetrasaccharide substrates of heparanase by glycosylation of disaccharides
- Synthetic heparin oligosaccharide microarrays for analysis of heparin-protein interactions
- Dexamethasone 21-sulfate sodium for use as a colon-specific prodrug
Other Notes
related product
Signal Word
Danger
Hazard Statements
Precautionary Statements
Hazard Classifications
Skin Corr. 1B
Storage Class Code
8A - Combustible corrosive hazardous materials
WGK
WGK 3
Personal Protective Equipment
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service




![1,8-Diazabicyclo[5.4.0]undec-7-ene 98%](/deepweb/assets/sigmaaldrich/product/structures/120/564/5b373e23-1624-489c-8efb-692de0f96ffb/640/5b373e23-1624-489c-8efb-692de0f96ffb.png)

