All Photos(1)
About This Item
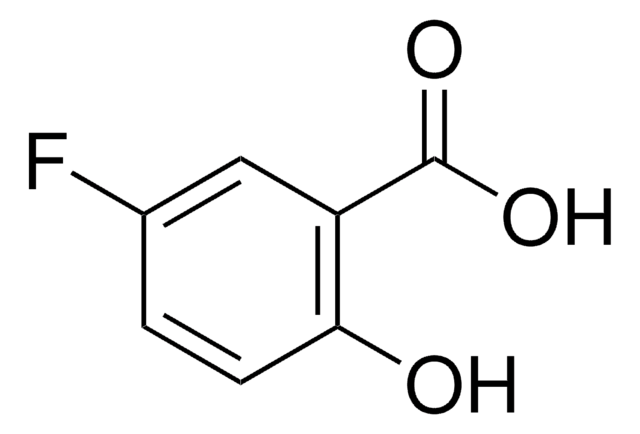
Empirical Formula (Hill Notation):
C13H18O7
CAS Number:
Molecular Weight:
286.28
MDL number:
UNSPSC Code:
12352201
PubChem Substance ID:
NACRES:
NA.22
Recommended Products
Quality Level
Assay
97%
form
solid
mp
133-172 °C
storage temp.
2-8°C
SMILES string
COc1ccc(O[C@@H]2O[C@H](CO)[C@@H](O)[C@H](O)[C@H]2O)cc1
InChI
1S/C13H18O7/c1-18-7-2-4-8(5-3-7)19-13-12(17)11(16)10(15)9(6-14)20-13/h2-5,9-17H,6H2,1H3/t9-,10-,11+,12-,13-/m1/s1
InChI key
SIXFVXJMCGPTRB-UJPOAAIJSA-N
Related Categories
Signal Word
Warning
Hazard Statements
Precautionary Statements
Hazard Classifications
Eye Irrit. 2
Storage Class Code
11 - Combustible Solids
WGK
WGK 3
Flash Point(F)
Not applicable
Flash Point(C)
Not applicable
Choose from one of the most recent versions:
Certificates of Analysis (COA)
Lot/Batch Number
Don't see the Right Version?
If you require a particular version, you can look up a specific certificate by the Lot or Batch number.
Already Own This Product?
Find documentation for the products that you have recently purchased in the Document Library.
G M Aerts et al.
Biochimica et biophysica acta, 660(2), 317-324 (1981-08-13)
The interaction of alcohols in the hydrolysis of aryl beta-D-glucopyranosides and aryl beta-D-xylopyranosides by beta-D-glucosidase (beta-D-glucoside glucohydrolase, EC 3.2.1.21) from Stachybotrys atra has been investigated. The results constitute support for the presence of a glycosyl-enzyme intermediate, formed during the first
Z Zhang et al.
Carbohydrate research, 295, 41-55 (1996-12-13)
p-Methoxyphenyl (pMP) beta-D-glycopyranosides (Glc, Gal, GlcNPhth, GalNPhth, GlcNTroc, Gal beta 4Glc, Gal alpha 4Gal) were prepared from the corresponding 1-O-acetyl sugars in 79-90% yield, using boron trifluoride etherate as promoter. Treatment of the pMP glycosides with acyl chlorides or bromides
Our team of scientists has experience in all areas of research including Life Science, Material Science, Chemical Synthesis, Chromatography, Analytical and many others.
Contact Technical Service